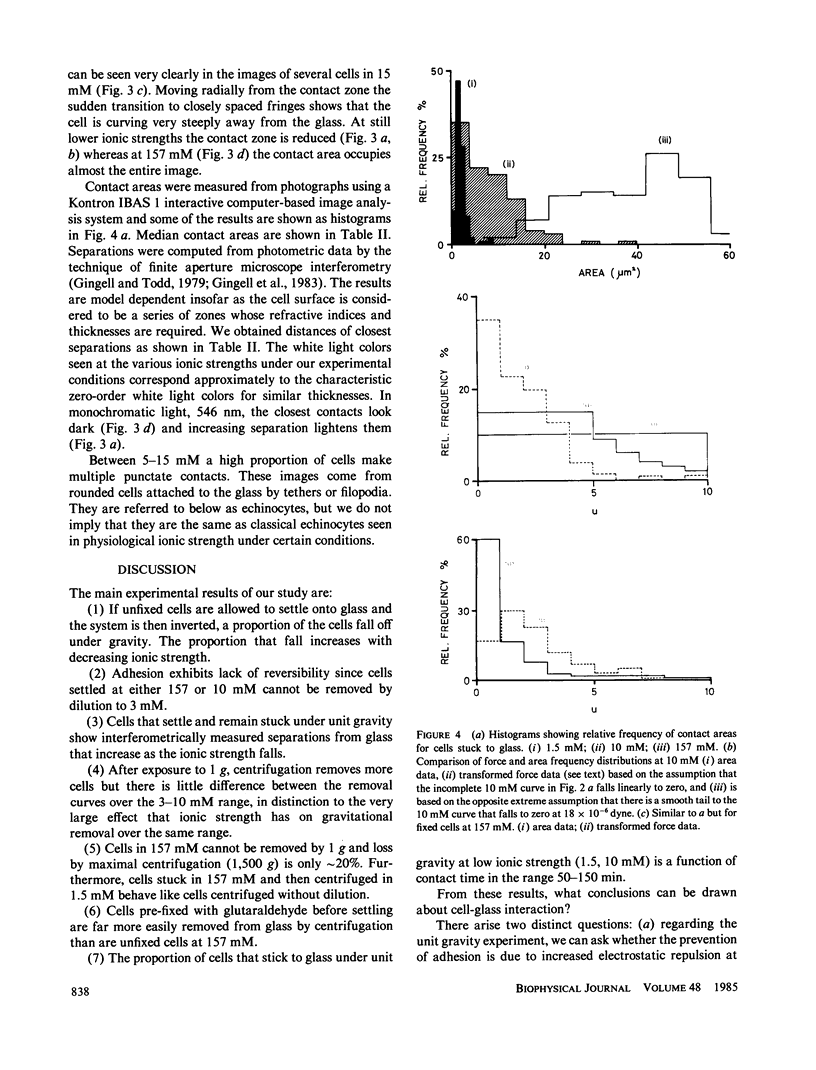
Abstract
We have studied the detachment of unfixed red cells from glass coverslips under unit gravity and by centrifugation in buffered isotonic solutions over a range of ionic strengths. Cell-glass contact areas and separation distances were measured by quantitative interference reflection microscopy. Detachment under unit gravity is highly dependent on ionic strength: dilution increases electrostatic repulsion and greatly reduces the proportion of adherent cells. However, even at 1.5 mM some cells stick. Over the range 3-110 mM such adherent cells are progressively removed by increasing centrifugal forces, but in a manner virtually independent of ionic strength. This fact, together with the irreversibility of pre-adherent cells as ionic strength is progressively reduced, as well as the resistance of cells to lateral shearing forces, provide evidence sufficient to reject the notion of secondary minimum adhesion for unfixed cells at any ionic strength down to 1.5 mM. We conclude that all unfixed cells that stick at ionic strengths from 157 to 1.5 mM make molecular contacts with glass. Comparison with long range force calculations suggests that to penetrate the electrostatic repulsion barrier the contact regions are unlikely to have average surface properties. A new method that compares frequency distributions of contact areas with responses to detachment forces shows that detachment forces are not linearly related to contact areas. This lack of relationship is less clearly evident for rigid glutaraldehyde-fixed cells and may therefore depend on the degree of cellular deformability.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Canham P. B., Burton A. C. Distribution of size and shape in populations of normal human red cells. Circ Res. 1968 Mar;22(3):405–422. doi: 10.1161/01.res.22.3.405. [DOI] [PubMed] [Google Scholar]
- Corry W. D., Defendi V. Centrifugal assessment of cell adhesion. J Biochem Biophys Methods. 1981 Jan;4(1):29–38. doi: 10.1016/0165-022x(81)90003-8. [DOI] [PubMed] [Google Scholar]
- DANON D., MARIKOVSKY V. DETERMINATION OF DENSITY DISTRIBUTION OF RED CELL POPULATION. J Lab Clin Med. 1964 Oct;64:668–674. [PubMed] [Google Scholar]
- Donath E., Gingell D. A sharp cell surface conformational transition at low ionic strength changes the nature of the adhesion of enzyme-treated red blood cells to a hydrocarbon interface. J Cell Sci. 1983 Sep;63:113–124. doi: 10.1242/jcs.63.1.113. [DOI] [PubMed] [Google Scholar]
- George J. N., Weed R. I., Reed C. F. Adhesion of human erythrocytes to glass: the nature of the interaction and the effect of serum and plasma. J Cell Physiol. 1971 Feb;77(1):51–59. doi: 10.1002/jcp.1040770107. [DOI] [PubMed] [Google Scholar]
- Gingell D., Fornes J. A. Interaction of red blood cells with a polarized electrode: evidence of long-range intermolecular forces. Biophys J. 1976 Oct;16(10):1131–1153. doi: 10.1016/S0006-3495(76)85763-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingell D., Todd I. Adhesion of red blood cells to charged interfaces between immiscible liquids. A new method. J Cell Sci. 1975 Jul;18(2):227–239. doi: 10.1242/jcs.18.2.227. [DOI] [PubMed] [Google Scholar]
- Gingell D., Todd I. Interference reflection microscopy. A quantitative theory for image interpretation and its application to cell-substratum separation measurement. Biophys J. 1979 Jun;26(3):507–526. doi: 10.1016/S0006-3495(79)85268-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingell D., Todd I. Red blood cell adhesion. II. Interferometric examination of the interaction with hydrocarbon oil and glass. J Cell Sci. 1980 Feb;41:135–149. doi: 10.1242/jcs.41.1.135. [DOI] [PubMed] [Google Scholar]
- Mohandas N., Hochmuth R. M., Spaeth E. E. Adhesion of red cells to foreign surfaces in the presence of flow. J Biomed Mater Res. 1974 Mar;8(2):119–136. doi: 10.1002/jbm.820080203. [DOI] [PubMed] [Google Scholar]
- Parsegian V. A., Gingell D. Red blood cell adhesion. III. Analysis of forces. J Cell Sci. 1980 Feb;41:151–157. doi: 10.1242/jcs.41.1.151. [DOI] [PubMed] [Google Scholar]
- Voigt A., Wolf H., Lauckner S., Neumann G., Becker R., Richter L. Electrokinetic properties of polymer and glass surfaces in aqueous solutions: experimental evidence for swollen surface layers. Biomaterials. 1983 Oct;4(4):299–304. doi: 10.1016/0142-9612(83)90032-7. [DOI] [PubMed] [Google Scholar]
- Wolf H., Gingell D. Conformational response of the glycocalyx to ionic strength and interaction with modified glass surfaces: study of live red cells by interferometry. J Cell Sci. 1983 Sep;63:101–112. doi: 10.1242/jcs.63.1.101. [DOI] [PubMed] [Google Scholar]