Abstract
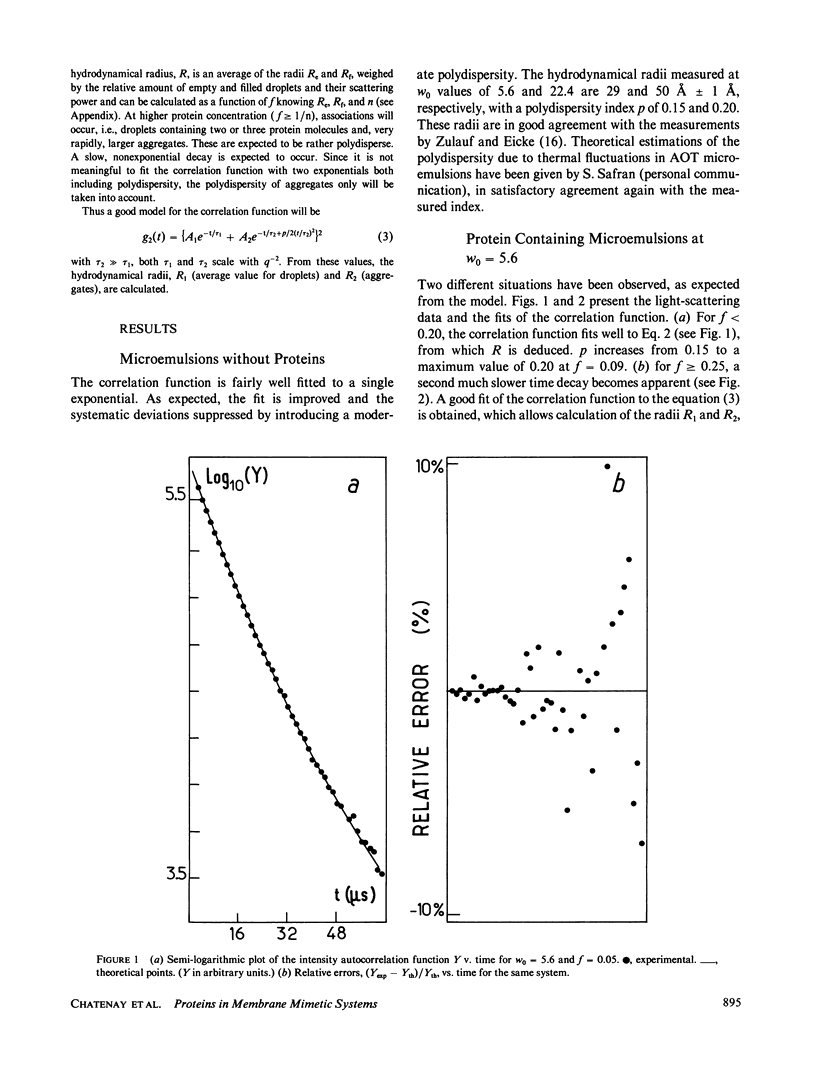
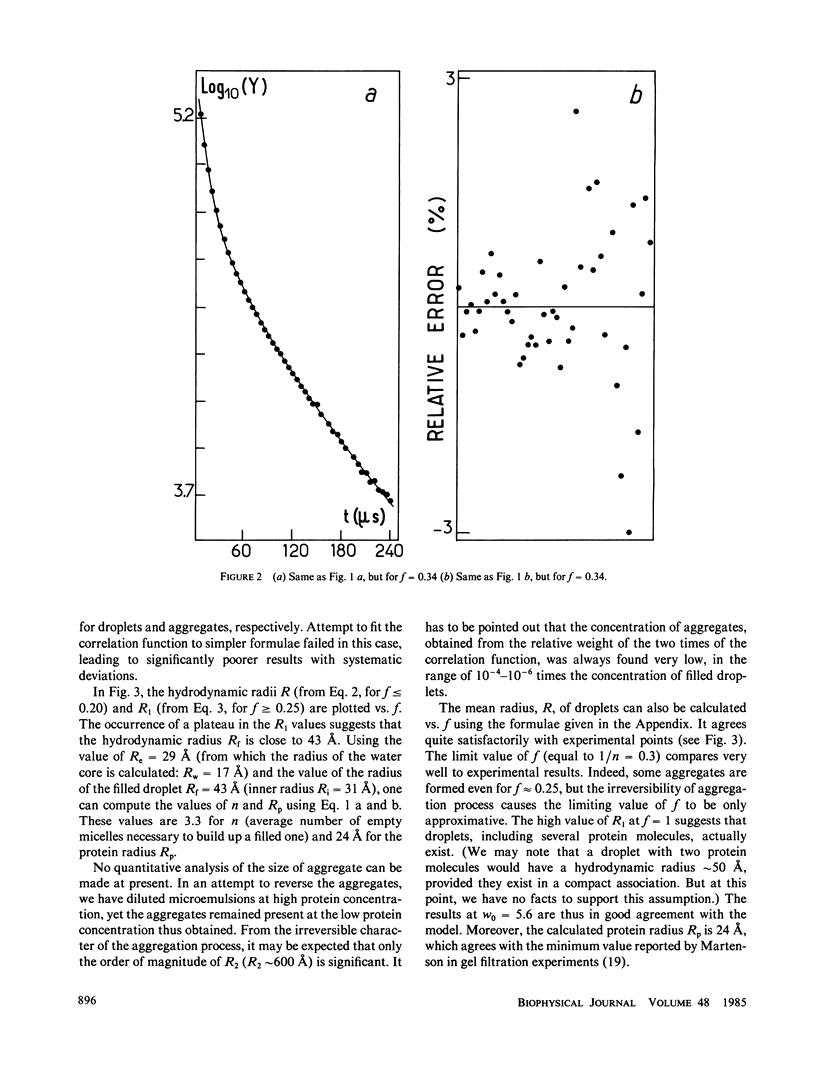
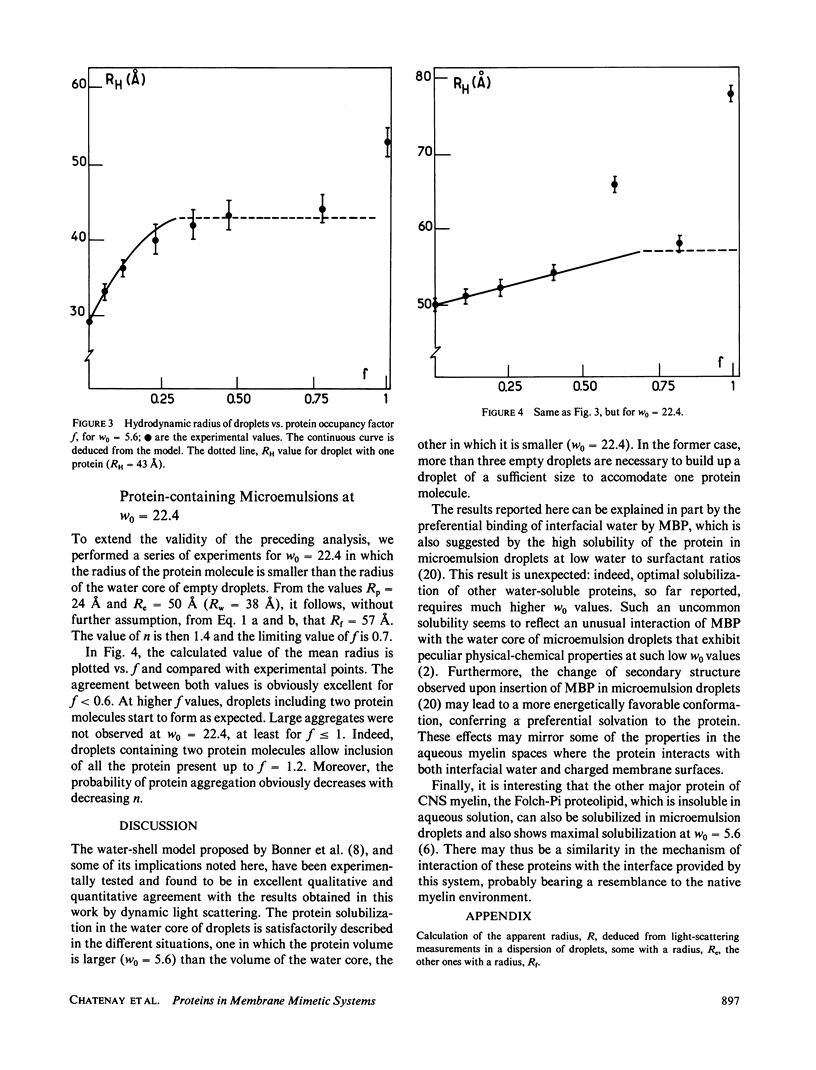
The insertion of myelin basic protein into microemulsion droplets of sodium bis (2-ethylhexyl) sulfosuccinate (AOT) has been studied by quasi-elastic light scattering. Measurements were made at both low and high molar ratios of water to surfactant, as a function of protein occupancy. The hydrodynamic radii of filled and empty droplets were experimentally evaluated. These were compared to values calculated using a water shell model of protein encapsulation, and excellent agreement was obtained. At low molar ratio of water to surfactant (w0 = 5.6), the hydrodynamic radius of filled droplets is significantly larger than the radius of empty ones. Under these conditions, about three empty (water-filled) droplets are required to build up a droplet of sufficient size to accommodate a single protein molecule. At maximum solubilization, which occurs at w0 = 5.6, a small fraction of droplets are found containing protein aggregates. In contrast, results at high values of w0 (22.4) reveal radii for empty and occupied droplets of comparable dimension, and the absence of aggregates. The results are discussed in terms of the model and the mechanism of interaction of this protein with the aqueous interfaces provided by these membrane-mimetic systems.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Deibler G. E., Martenson R. E., Kies M. W. Large scale preparation of myelin basic protein from central nervous tissue of several mammalian species. Prep Biochem. 1972;2(2):139–165. doi: 10.1080/00327487208061467. [DOI] [PubMed] [Google Scholar]
- Eylar E. H. Amino acid sequence of the basic protein of the myelin membrane. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1425–1431. doi: 10.1073/pnas.67.3.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imre V. E., Luisi P. L. Solubilization and condensed packaging of nucleic acids in reversed micelles. Biochem Biophys Res Commun. 1982 Jul 30;107(2):538–545. doi: 10.1016/0006-291x(82)91525-x. [DOI] [PubMed] [Google Scholar]
- Martenson R. E. The use of gel filtration to follow conformational changes in proteins. Conformational flexibility of bovine myelin basic protein. J Biol Chem. 1978 Dec 25;253(24):8887–8893. [PubMed] [Google Scholar]
- Martinek K., Levashov A. V., Klyachko N. L., Pantin V. I., Berezin I. V. The principles of enzyme stabilization. VI. Catalysis by water-soluble enzymes entrapped into reversed micelles of surfactants in organic solvents. Biochim Biophys Acta. 1981 Jan 15;657(1):277–294. doi: 10.1016/0005-2744(81)90151-0. [DOI] [PubMed] [Google Scholar]
- Wolf R., Luisi P. L. Micellar solubilization of enzymes in hydrocarbon solvents. Enzymatic activity and spectroscopic properties of ribonuclease in N-octane. Biochem Biophys Res Commun. 1979 Jul 12;89(1):209–217. doi: 10.1016/0006-291x(79)90965-3. [DOI] [PubMed] [Google Scholar]


