Abstract
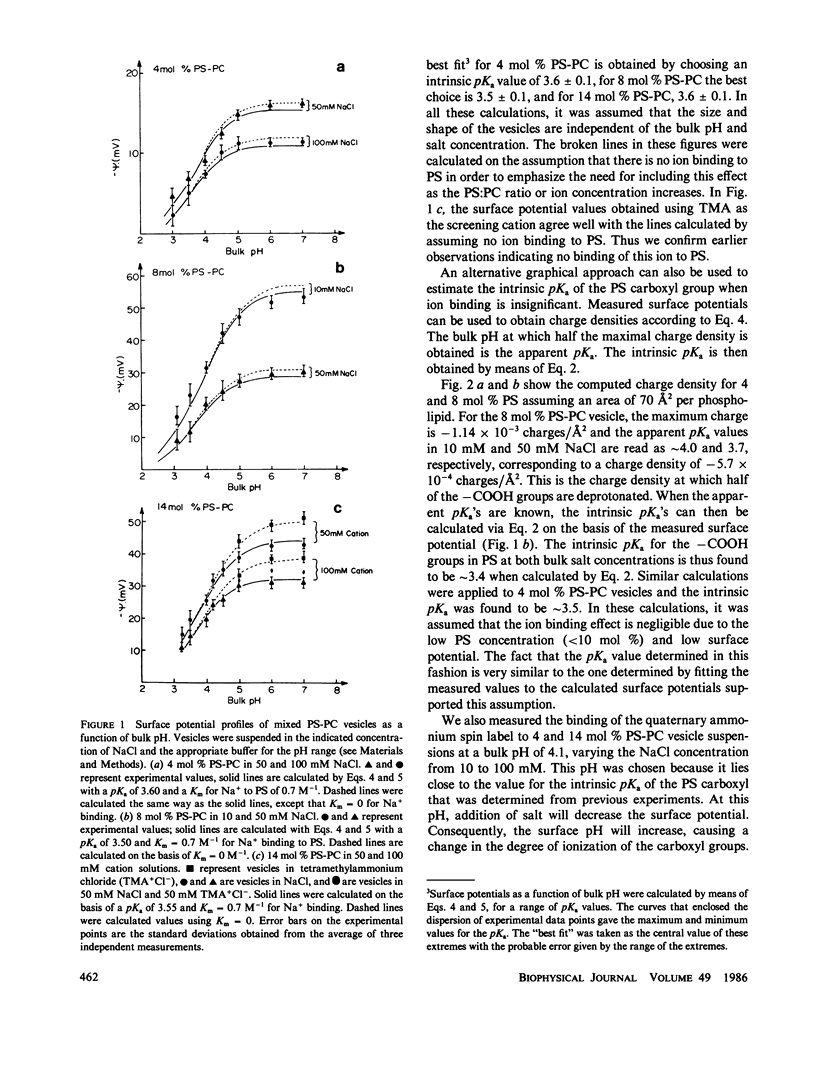
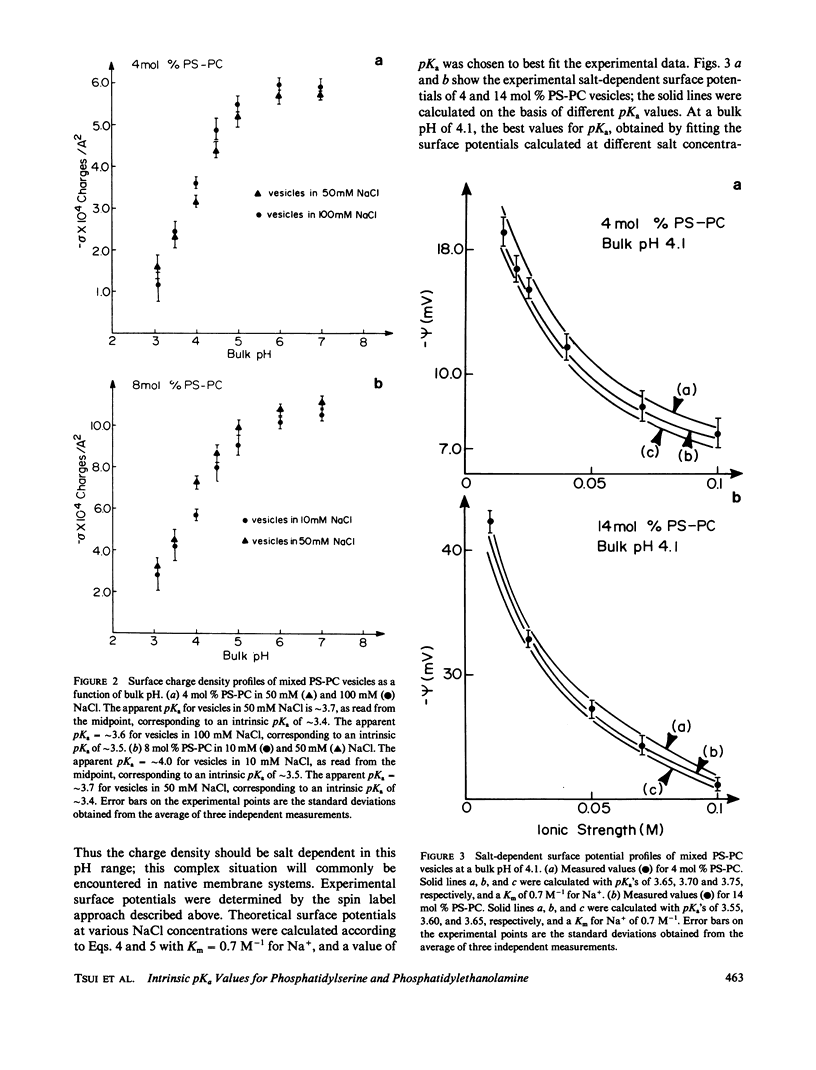
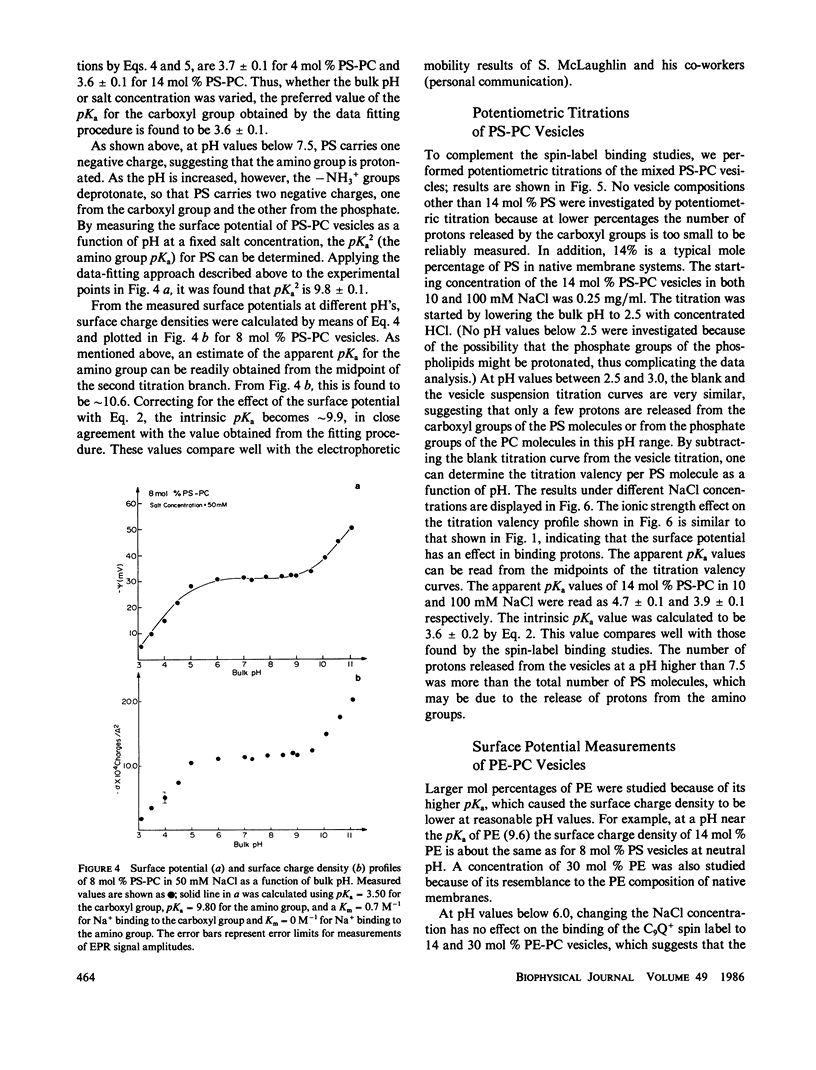
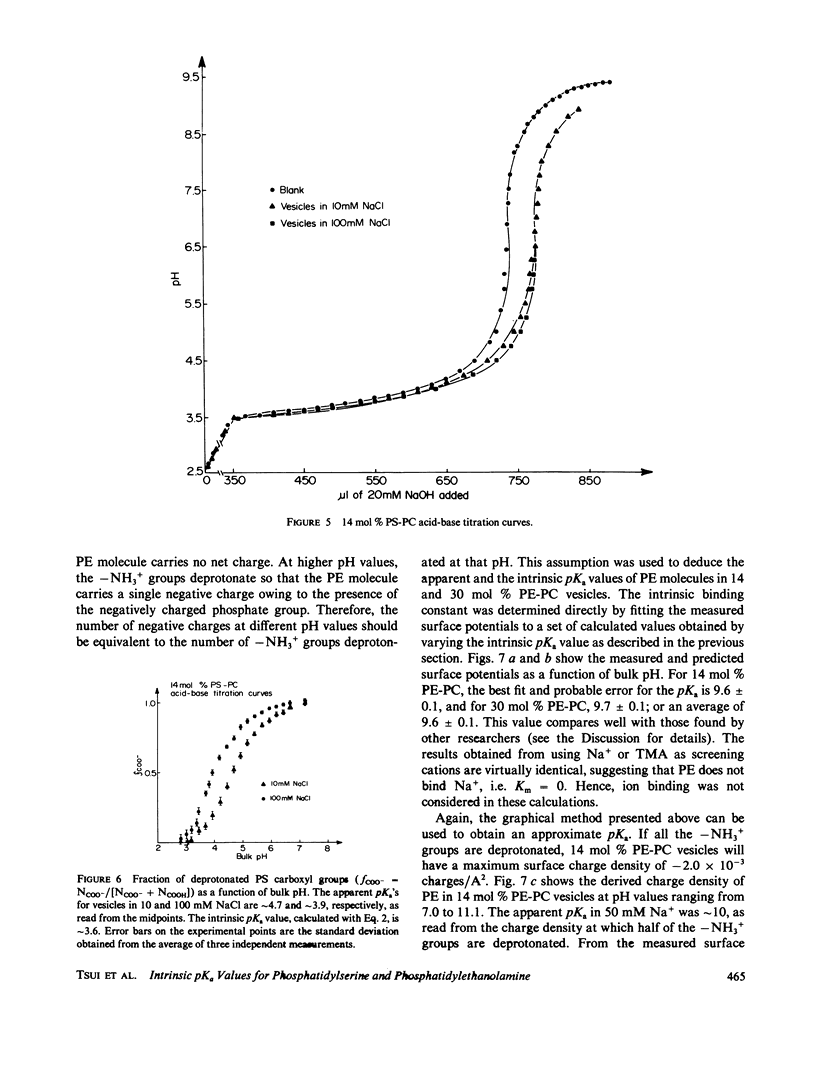
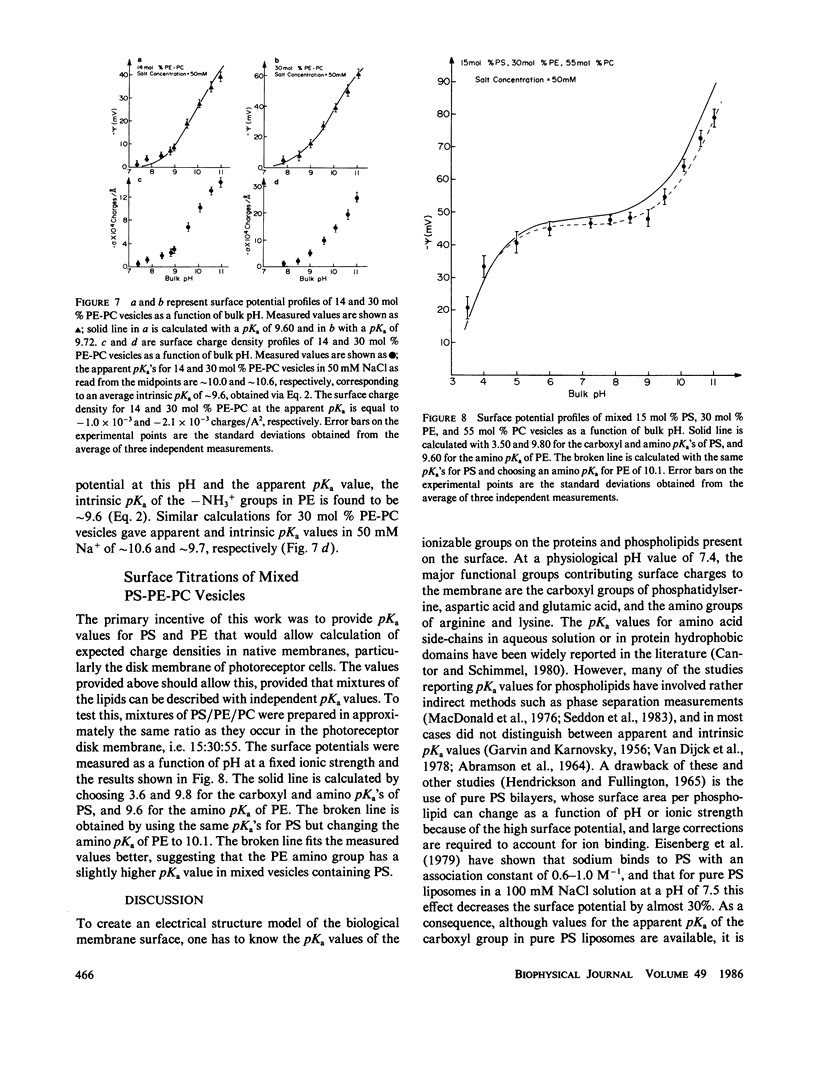
Potentiometric titrations and surface potential measurements have been used to determine the intrinsic pKa values of both the carboxyl and amino groups of phosphatidylserine (PS) in mixed vesicles of PS and phosphatidylcholine (PC), and also of the amino group of phosphatidylethanolamine (PE) in mixed PE-PC vesicles. The pKa of the carboxyl group of PS in liposomes with different PS/PC lipid ratios measured by the two different methods is 3.6 +/- 0.1, and the pKa of its amino group is 9.8 +/- 0.1. The pKa of the amino group of PE in PE-PC vesicles, determined solely by surface potential measurements, is 9.6 +/- 0.1. These pKa values are independent of the aqueous phase ionic strength and of the effect of the liposome's surface potential due to the presence of these partially charged lipids.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAMSON M. B., KATZMAN R., GREGOR H. P. AQUEOUS DISPERSIONS OF PHOSPHATIDYLSERINE. IONIC PROPERTIES. J Biol Chem. 1964 Jan;239:70–76. [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Cafiso D. S., Hubbell W. L. EPR determination of membrane potentials. Annu Rev Biophys Bioeng. 1981;10:217–244. doi: 10.1146/annurev.bb.10.060181.001245. [DOI] [PubMed] [Google Scholar]
- Castle J. D., Hubbell W. L. Estimation of membrane surface potential and charge density from the phase equilibrium of a paramagnetic amphiphile. Biochemistry. 1976 Nov 2;15(22):4818–4831. doi: 10.1021/bi00667a011. [DOI] [PubMed] [Google Scholar]
- Cevc G., Watts A., Marsh D. Titration of the phase transition of phosphatidylserine bilayer membranes. Effects of pH, surface electrostatics, ion binding, and head-group hydration. Biochemistry. 1981 Aug 18;20(17):4955–4965. doi: 10.1021/bi00520a023. [DOI] [PubMed] [Google Scholar]
- Chabre M. X-ray diffraction studies of retinal rods. I. Structure of the disc membrane, effect of illumination. Biochim Biophys Acta. 1975 Mar 25;382(3):322–335. doi: 10.1016/0005-2736(75)90274-6. [DOI] [PubMed] [Google Scholar]
- Corless J. M. Lamellar structure of bleached and unbleached rod photoreceptor membranes. Nature. 1972 May 26;237(5352):229–231. doi: 10.1038/237229a0. [DOI] [PubMed] [Google Scholar]
- Eisenberg M., Gresalfi T., Riccio T., McLaughlin S. Adsorption of monovalent cations to bilayer membranes containing negative phospholipids. Biochemistry. 1979 Nov 13;18(23):5213–5223. doi: 10.1021/bi00590a028. [DOI] [PubMed] [Google Scholar]
- GARVIN J. E., KARNOVSKY M. L. The titration of some phosphatides and related compounds in a non-aqueous medium. J Biol Chem. 1956 Jul;221(1):211–222. [PubMed] [Google Scholar]
- Hauser H., Shipley G. G. Interactions of divalent cations with phosphatidylserine bilayer membranes. Biochemistry. 1984 Jan 3;23(1):34–41. doi: 10.1021/bi00296a006. [DOI] [PubMed] [Google Scholar]
- Hendrickson H. S., Fullington J. G. Stabilities of metal complexes of phospholipids: Ca(II), Mg(II), and Ni(II) complexes of phosphatidylserine and triphosphoinositide. Biochemistry. 1965 Aug;4(8):1599–1605. doi: 10.1021/bi00884a021. [DOI] [PubMed] [Google Scholar]
- Hubbell W. L., Metcalfe J. C., Metcalfe S. M., McConnell H. M. The interaction of small molecules with spin-labelled erythrocyte membranes. Biochim Biophys Acta. 1970 Dec 1;219(2):415–427. doi: 10.1016/0005-2736(70)90219-1. [DOI] [PubMed] [Google Scholar]
- Johnson S. M. The effect of charge and cholesterol on the size and thickness of sonicated phospholipid vesicles. Biochim Biophys Acta. 1973 Apr 25;307(1):27–41. doi: 10.1016/0005-2736(73)90022-9. [DOI] [PubMed] [Google Scholar]
- Lelkes P. I., Miller I. R. Perturbations of membrane structure by optical probes: I. Location and structural sensitivity of merocyanine 540 bound to phospholipid membranes. J Membr Biol. 1980 Jan 31;52(1):1–15. doi: 10.1007/BF01869001. [DOI] [PubMed] [Google Scholar]
- MacDonald R. C., Simon S. A., Baer E. Ionic influences on the phase transition of dipalmitoylphosphatidylserine. Biochemistry. 1976 Feb 24;15(4):885–891. doi: 10.1021/bi00649a025. [DOI] [PubMed] [Google Scholar]
- Papahadjopoulos D. Surface properties of acidic phospholipids: interaction of monolayers and hydrated liquid crystals with uni- and bi-valent metal ions. Biochim Biophys Acta. 1968 Sep 17;163(2):240–254. doi: 10.1016/0005-2736(68)90103-x. [DOI] [PubMed] [Google Scholar]
- SINGLETON W. S., GRAY M. S., BROWN M. L., WHITE J. L. CHROMATOGRAPHICALLY HOMOGENEOUS LECITHIN FROM EGG PHOSPHOLIPIDS. J Am Oil Chem Soc. 1965 Jan;42:53–56. doi: 10.1007/BF02558256. [DOI] [PubMed] [Google Scholar]
- Sculley M. J., Duniec J. T., Thorne S. W., Chow W. S., Boardman N. K. The stacking of chloroplast thylakoids. Quantitative analysis of the balance of forces between thylakoid membranes of chloroplasts, and the role of divalent cations. Arch Biochem Biophys. 1980 Apr 15;201(1):339–346. doi: 10.1016/0003-9861(80)90519-6. [DOI] [PubMed] [Google Scholar]
- Seddon J. M., Cevc G., Marsh D. Calorimetric studies of the gel-fluid (L beta-L alpha) and lamellar-inverted hexagonal (L alpha-HII) phase transitions in dialkyl- and diacylphosphatidylethanolamines. Biochemistry. 1983 Mar 1;22(5):1280–1289. doi: 10.1021/bi00274a045. [DOI] [PubMed] [Google Scholar]
- Szabo G., Eisenman G., McLaughlin S. G., Krasne S. Ionic probes of membrane structures. Ann N Y Acad Sci. 1972 Jun 20;195:273–290. [PubMed] [Google Scholar]
- van Dijck P. W., de Kruijff B., Verkleij A. J., van Deenen L. L., de Gier J. Comparative studies on the effects of pH and Ca2+ on bilayers of various negatively charged phospholipids and their mixtures with phosphatidylcholine. Biochim Biophys Acta. 1978 Sep 11;512(1):84–96. doi: 10.1016/0005-2736(78)90219-5. [DOI] [PubMed] [Google Scholar]


