Abstract
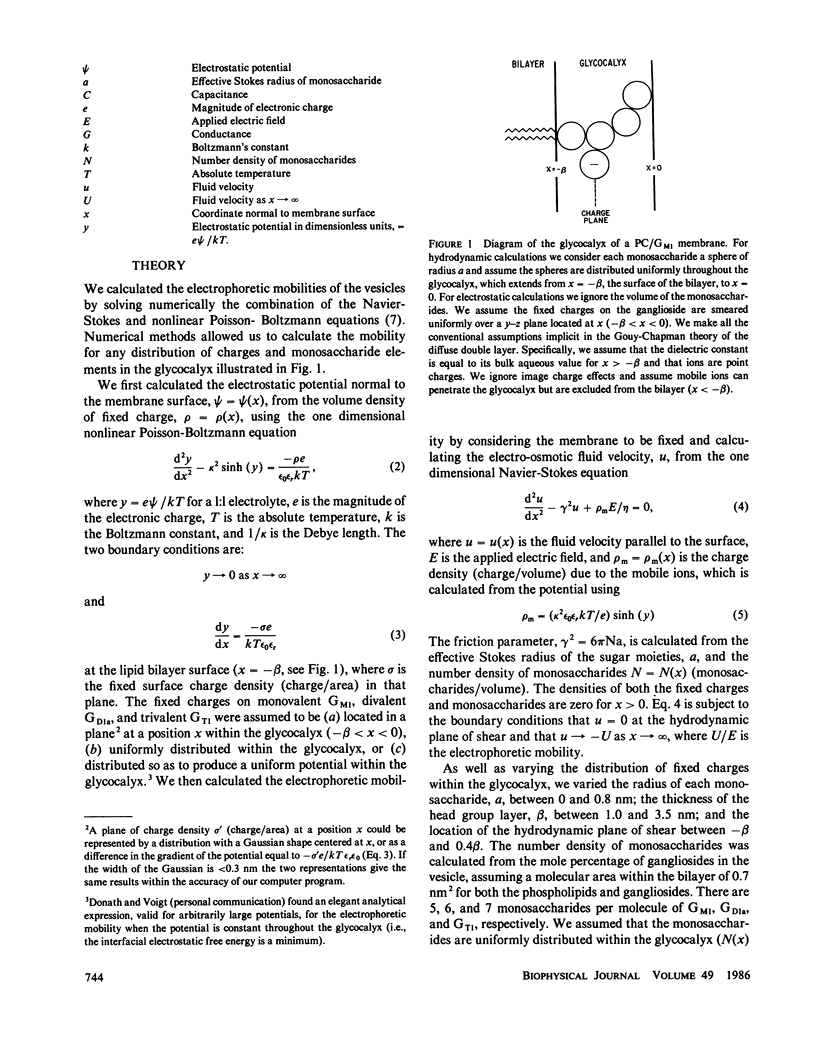
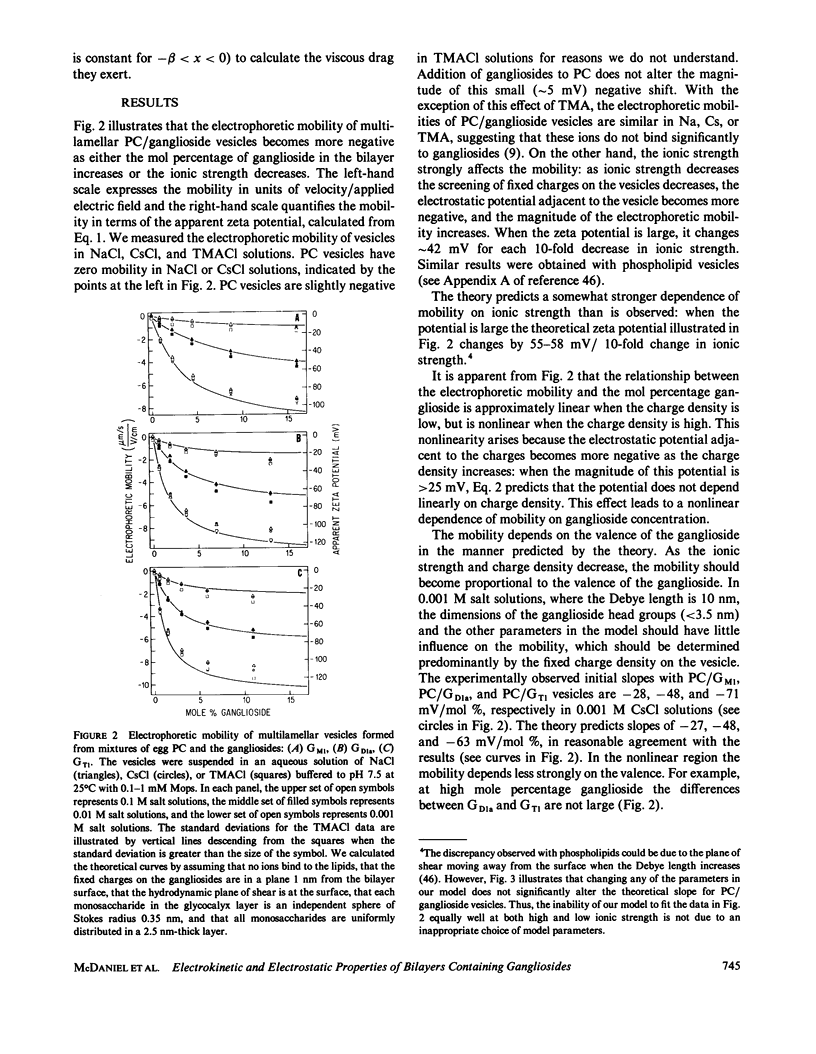
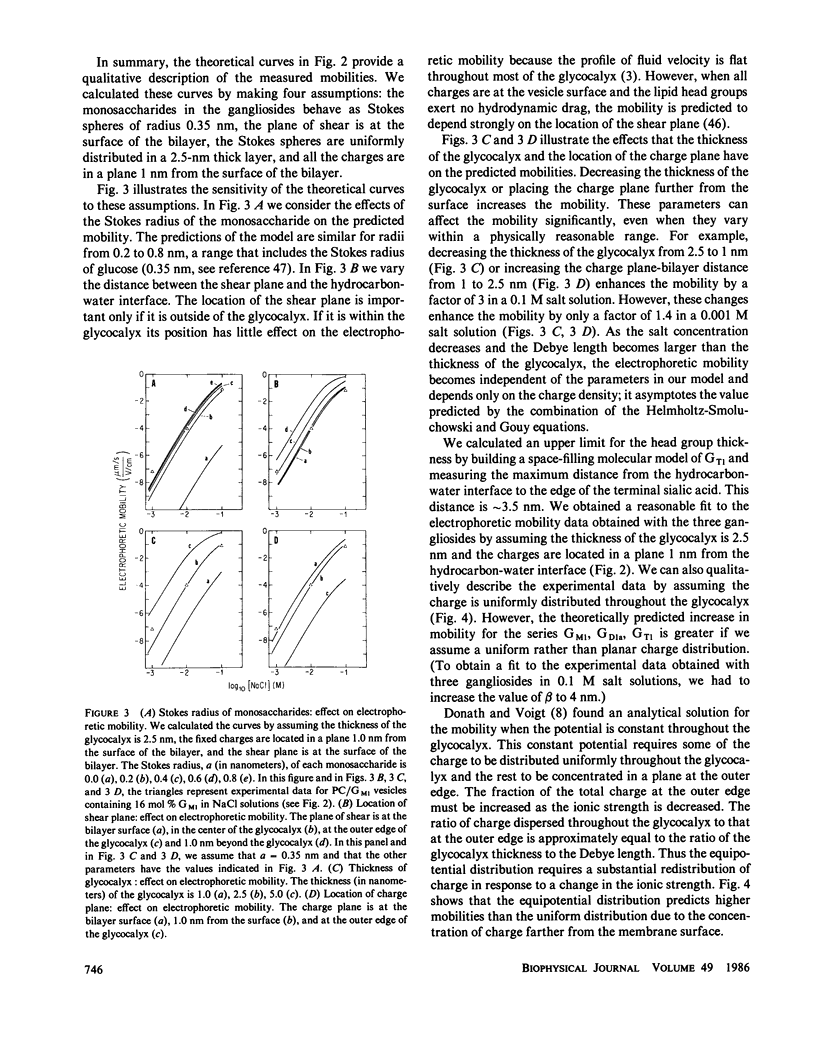
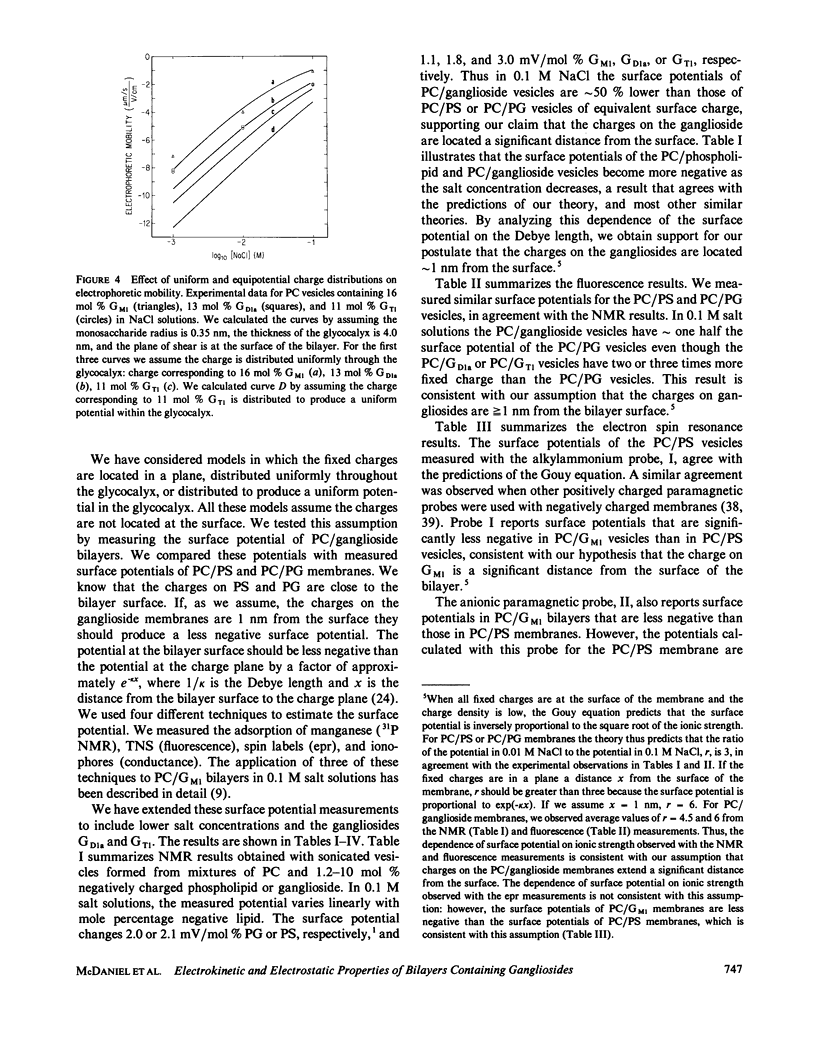
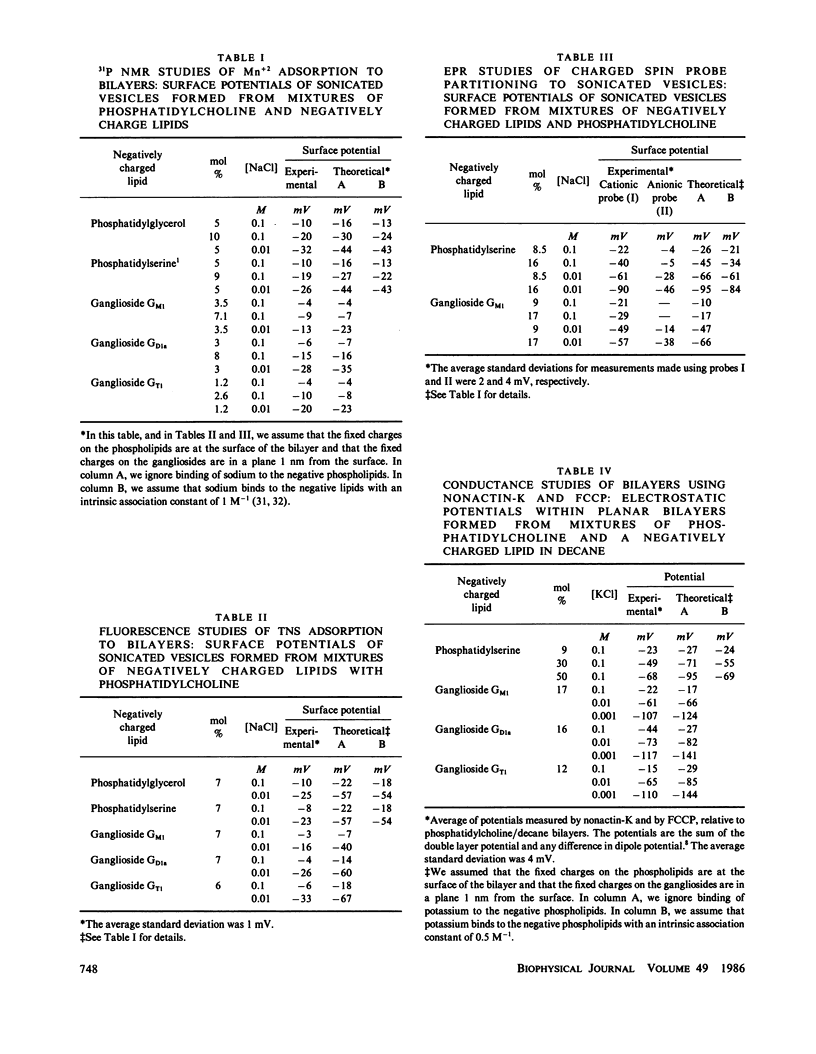
We formed vesicles from mixtures of egg phosphatidylcholine (PC) and the gangliosides GM1, GD1a, or GT1 to model the electrokinetic properties of biological membranes. The electrophoretic mobilities of the vesicles are similar in NaCl, CsCl, and TMACl solutions, suggesting that monovalent cations do not bind significantly to these gangliosides. If we assume the sialic acid groups on the gangliosides are located some distance from the surface of the vesicle and the sugar moieties exert hydrodynamic drag, we can describe the mobility data in 1, 10, and 100 mM monovalent salt solutions with a combination of the Navier-Stokes and nonlinear Poisson-Boltzmann equations. The values we assume for the thickness of the ganglioside head group and the location of the charge affect the theoretical predictions markedly, but the Stokes radius of each sugar and the location of the hydrodynamic shear plane do not. We obtain a reasonable fit to the mobility data by assuming that all ganglioside head groups project 2.5 nm from the bilayer and all fixed charges are in a plane 1 nm from the bilayer surface. We tested the latter assumption by estimating the surface potentials of PC/ganglioside bilayers using four techniques: we made 31P nuclear magnetic resonance, fluorescence, electron spin resonance, and conductance measurements. The results are qualitatively consistent with our assumption.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen O. S., Feldberg S., Nakadomari H., Levy S., McLaughlin S. Electrostatic interactions among hydrophobic ions in lipid bilayer membranes. Biophys J. 1978 Jan;21(1):35–70. doi: 10.1016/S0006-3495(78)85507-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Bach D., Miller I. R., Sela B. A. Calorimetric studies on various gangliosides and ganglioside-lipid interactions. Biochim Biophys Acta. 1982 Apr 7;686(2):233–239. doi: 10.1016/0005-2736(82)90117-1. [DOI] [PubMed] [Google Scholar]
- Barenholz Y., Gibbes D., Litman B. J., Goll J., Thompson T. E., Carlson R. D. A simple method for the preparation of homogeneous phospholipid vesicles. Biochemistry. 1977 Jun 14;16(12):2806–2810. doi: 10.1021/bi00631a035. [DOI] [PubMed] [Google Scholar]
- Bloomfield V., Dalton W. O., Van Holde K. E. Frictional coefficients of multisubunit structures. I. Theory. Biopolymers. 1967 Feb;5(2):135–148. doi: 10.1002/bip.1967.360050202. [DOI] [PubMed] [Google Scholar]
- Bremer E. G., Hakomori S., Bowen-Pope D. F., Raines E., Ross R. Ganglioside-mediated modulation of cell growth, growth factor binding, and receptor phosphorylation. J Biol Chem. 1984 Jun 10;259(11):6818–6825. [PubMed] [Google Scholar]
- Cafiso D. S., Hubbell W. L. EPR determination of membrane potentials. Annu Rev Biophys Bioeng. 1981;10:217–244. doi: 10.1146/annurev.bb.10.060181.001245. [DOI] [PubMed] [Google Scholar]
- Cafiso D. S., Hubbell W. L. Light-induced interfacial potentials in photoreceptor membranes. Biophys J. 1980 May;30(2):243–263. doi: 10.1016/S0006-3495(80)85092-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle J. D., Hubbell W. L. Estimation of membrane surface potential and charge density from the phase equilibrium of a paramagnetic amphiphile. Biochemistry. 1976 Nov 2;15(22):4818–4831. doi: 10.1021/bi00667a011. [DOI] [PubMed] [Google Scholar]
- Cheresh D. A., Reisfeld R. A., Varki A. P. O-acetylation of disialoganglioside GD3 by human melanoma cells creates a unique antigenic determinant. Science. 1984 Aug 24;225(4664):844–846. doi: 10.1126/science.6206564. [DOI] [PubMed] [Google Scholar]
- Cole K. S. Zeta potential and discrete vs. uniform surface charges. Biophys J. 1969 Mar;9(3):465–469. doi: 10.1016/S0006-3495(69)86397-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curatolo W., Small D. M., Shipley G. G. Phase behavior and structural characteristics of hydrated bovine brain gangliosides. Biochim Biophys Acta. 1977 Jul 4;468(1):11–20. doi: 10.1016/0005-2736(77)90147-x. [DOI] [PubMed] [Google Scholar]
- Donath E., Voigt A. Charge distribution within cell surface coats of single and interacting surfaces--a minimum free electrostatic energy approach. Conclusions for electrophoretic mobility measurements. J Theor Biol. 1983 Apr 21;101(4):569–584. doi: 10.1016/0022-5193(83)90016-4. [DOI] [PubMed] [Google Scholar]
- Eisenberg M., Gresalfi T., Riccio T., McLaughlin S. Adsorption of monovalent cations to bilayer membranes containing negative phospholipids. Biochemistry. 1979 Nov 13;18(23):5213–5223. doi: 10.1021/bi00590a028. [DOI] [PubMed] [Google Scholar]
- Felgner P. L., Freire E., Barenholz Y., Thompson T. E. Asymmetric incorporation of trisialoganglioside into dipalmitoylphosphatidylcholine vesicles. Biochemistry. 1981 Apr 14;20(8):2168–2172. doi: 10.1021/bi00511a015. [DOI] [PubMed] [Google Scholar]
- Fishman P. H., Brady R. O. Biosynthesis and function of gangliosides. Science. 1976 Nov 26;194(4268):906–915. doi: 10.1126/science.185697. [DOI] [PubMed] [Google Scholar]
- Fishman P. H. Role of membrane gangliosides in the binding and action of bacterial toxins. J Membr Biol. 1982;69(2):85–97. doi: 10.1007/BF01872268. [DOI] [PubMed] [Google Scholar]
- Gaffney B. J., Mich R. J. Letter: A new measurement of surface charge in model and biological lipid membranes. J Am Chem Soc. 1976 May 12;98(10):3044–3045. doi: 10.1021/ja00426a076. [DOI] [PubMed] [Google Scholar]
- Ghidoni R., Sonnino S., Masserini M., Orlando P., Tettamanti G. Specific tritium labeling of gangliosides at the 3-position of sphingosines. J Lipid Res. 1981 Nov;22(8):1286–1295. [PubMed] [Google Scholar]
- Hakomori S. Glycosphingolipids in cellular interaction, differentiation, and oncogenesis. Annu Rev Biochem. 1981;50:733–764. doi: 10.1146/annurev.bi.50.070181.003505. [DOI] [PubMed] [Google Scholar]
- Harris R. A., Groh G. I., Baxter D. M., Hitzemann R. J. Gangliosides enhance the membrane actions of ethanol and pentobarbital. Mol Pharmacol. 1984 May;25(3):410–417. [PubMed] [Google Scholar]
- Heinrich R., Gaestel M., Glaser R. The electric potential profile across the erythrocyte membrane. J Theor Biol. 1982 May 21;96(2):211–231. doi: 10.1016/0022-5193(82)90222-3. [DOI] [PubMed] [Google Scholar]
- Hill M. W., Lester R. Mixtures of gangliosides and phosphatidylcholine in aqueous dispersions. Biochim Biophys Acta. 1972 Sep 1;282(1):18–30. doi: 10.1016/0005-2736(72)90307-0. [DOI] [PubMed] [Google Scholar]
- Klenk E., Georgias L. Uber zwei weitere Komponenten des Gemischs der Gehirnganglioside. Hoppe Seylers Z Physiol Chem. 1967 Oct;348(10):1261–1267. [PubMed] [Google Scholar]
- Lau A., McLaughlin A., McLaughlin S. The adsorption of divalent cations to phosphatidylglycerol bilayer membranes. Biochim Biophys Acta. 1981 Jul 20;645(2):279–292. doi: 10.1016/0005-2736(81)90199-1. [DOI] [PubMed] [Google Scholar]
- Ledeen R. W., Yu R. K., Eng L. F. Gangliosides of human myelin: sialosylgalactosylceramide (G7) as a major component. J Neurochem. 1973 Oct;21(4):829–839. doi: 10.1111/j.1471-4159.1973.tb07527.x. [DOI] [PubMed] [Google Scholar]
- Maggio B., Ariga T., Sturtevant J. M., Yu R. K. Thermotropic behavior of glycosphingolipids in aqueous dispersions. Biochemistry. 1985 Feb 26;24(5):1084–1092. doi: 10.1021/bi00326a003. [DOI] [PubMed] [Google Scholar]
- McDaniel R. V., McIntosh T. J. X-Ray Diffraction Studies of the Cholera Toxin receptor, G(M1). Biophys J. 1986 Jan;49(1):94–96. doi: 10.1016/s0006-3495(86)83606-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel R. V., McLaughlin A., Winiski A. P., Eisenberg M., McLaughlin S. Bilayer membranes containing the ganglioside GM1: models for electrostatic potentials adjacent to biological membranes. Biochemistry. 1984 Sep 25;23(20):4618–4624. doi: 10.1021/bi00315a016. [DOI] [PubMed] [Google Scholar]
- McLaughlin A. C. Phosphorus-31 and carbon-13 nuclear magnetic resonance studies of divalent cation binding to phosphatidylserine membranes: use of cobalt as a paramagnetic probe. Biochemistry. 1982 Sep 28;21(20):4879–4885. doi: 10.1021/bi00263a008. [DOI] [PubMed] [Google Scholar]
- McLaughlin A., Eng W. K., Vaio G., Wilson T., McLaughlin S. Dimethonium, a divalent cation that exerts only a screening effect on the electrostatic potential adjacent to negatively charged phospholipid bilayer membranes. J Membr Biol. 1983;76(2):183–193. doi: 10.1007/BF02000618. [DOI] [PubMed] [Google Scholar]
- McLaughlin S. G., Szabo G., Eisenman G., Ciani S. M. Surface charge and the conductance of phospholipid membranes. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1268–1275. doi: 10.1073/pnas.67.3.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin S. G., Szabo G., Eisenman G. Divalent ions and the surface potential of charged phospholipid membranes. J Gen Physiol. 1971 Dec;58(6):667–687. doi: 10.1085/jgp.58.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin S., Mulrine N., Gresalfi T., Vaio G., McLaughlin A. Adsorption of divalent cations to bilayer membranes containing phosphatidylserine. J Gen Physiol. 1981 Apr;77(4):445–473. doi: 10.1085/jgp.77.4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitaku S., Jippo T., Kataoka R. Thermodynamic properties of the lipid bilayer transition. Pseudocritical phenomena. Biophys J. 1983 May;42(2):137–144. doi: 10.1016/S0006-3495(83)84379-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers M., Wortman C., Freire E. Modulation of neuraminidase activity by the physical state of phospholipid bilayers containing gangliosides Gd1a and Gt1b. Biochemistry. 1984 Mar 27;23(7):1442–1448. doi: 10.1021/bi00302a016. [DOI] [PubMed] [Google Scholar]
- Nelson A. P., McQuarrie D. A. The effect of discrete charges on the electrical properties of a membrane. I. J Theor Biol. 1975 Nov;55(1):13–27. doi: 10.1016/s0022-5193(75)80106-8. [DOI] [PubMed] [Google Scholar]
- Parsegian V. A. Possible modulation of reactions on the cell surface by changes in electrostatic potential that accompany cell contact. Ann N Y Acad Sci. 1974;238:362–371. doi: 10.1111/j.1749-6632.1974.tb26804.x. [DOI] [PubMed] [Google Scholar]
- Peters M. W., Mehlhorn I. E., Barber K. R., Grant C. W. Evidence of a distribution difference between two gangliosides in bilayer membranes. Biochim Biophys Acta. 1984 Dec 19;778(3):419–428. doi: 10.1016/0005-2736(84)90389-4. [DOI] [PubMed] [Google Scholar]
- Poss A., Deleers M., Ruysschaert J. M. Evidence for a specific interaction between GT1 ganglioside incorporated into bilayer membranes and thyrotropin. FEBS Lett. 1978 Feb 15;86(2):160–162. doi: 10.1016/0014-5793(78)80553-5. [DOI] [PubMed] [Google Scholar]
- Puskin J. S. Divalent cation binding to phospholipids: an EPR study. J Membr Biol. 1977 Jun 24;35(1):39–55. doi: 10.1007/BF01869939. [DOI] [PubMed] [Google Scholar]
- SVENNERHOLM L. CHROMATOGRAPHIC SEPARATION OF HUMAN BRAIN GANGLIOSIDES. J Neurochem. 1963 Sep;10:613–623. doi: 10.1111/j.1471-4159.1963.tb08933.x. [DOI] [PubMed] [Google Scholar]
- Sabel B. A., Slavin M. D., Stein D. G. GM1 ganglioside treatment facilitates behavioral recovery from bilateral brain damage. Science. 1984 Jul 20;225(4659):340–342. doi: 10.1126/science.6740316. [DOI] [PubMed] [Google Scholar]
- Sela B. A., Bach D. Calorimetric studies on the interaction of gangliosides with phospholipids and myelin basic protein. Biochim Biophys Acta. 1984 Apr 11;771(2):177–182. doi: 10.1016/0005-2736(84)90530-3. [DOI] [PubMed] [Google Scholar]
- Sharom F. J., Grant C. W. A model for ganglioside behaviour in cell membranes. Biochim Biophys Acta. 1978 Feb 21;507(2):280–293. doi: 10.1016/0005-2736(78)90423-6. [DOI] [PubMed] [Google Scholar]
- Sharp K. A., Brooks D. E. Calculation of the electrophoretic mobility of a particle bearing bound polyelectrolyte using the nonlinear poisson-boltzmann equation. Biophys J. 1985 Apr;47(4):563–566. doi: 10.1016/S0006-3495(85)83951-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui B., McCluer R. H. Lipid components of sialosylgalactosylceramide of human brain. J Lipid Res. 1968 May;9(3):366–370. [PubMed] [Google Scholar]
- Thompson T. E., Allietta M., Brown R. E., Johnson M. L., Tillack T. W. Organization of ganglioside GM1 in phosphatidylcholine bilayers. Biochim Biophys Acta. 1985 Jul 25;817(2):229–237. doi: 10.1016/0005-2736(85)90024-0. [DOI] [PubMed] [Google Scholar]
- Tillack T. W., Wong M., Allietta M., Thompson T. E. Organization of the glycosphingolipid asialo-GM1 in phosphatidylcholine bilayers. Biochim Biophys Acta. 1982 Oct 7;691(2):261–273. doi: 10.1016/0005-2736(82)90415-1. [DOI] [PubMed] [Google Scholar]
- Tosteson M. T., Tosteson D. C., Rubnitz J. Cholera toxin interactions with lipid bilayers. Acta Physiol Scand Suppl. 1980;481:21–25. [PubMed] [Google Scholar]
- Tsao Y. S., Huang L. Sendai virus induced leakage of liposomes containing gangliosides. Biochemistry. 1985 Feb 26;24(5):1092–1098. doi: 10.1021/bi00326a004. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y. A virial expansion for discrete charges buried in a membrane. Biophys J. 1978 Nov;24(2):561–567. doi: 10.1016/S0006-3495(78)85402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usai C., Robello M., Gambale F., Marchetti C. Effect of gangliosides on phospholipid bilayers: a study with the lipophilic ions relaxation method. J Membr Biol. 1984;82(1):15–23. doi: 10.1007/BF01870728. [DOI] [PubMed] [Google Scholar]
- Wang C. C., Bruner L. J. Evidence for a discrete charge effect within lipid bilayer membranes. Biophys J. 1978 Dec;24(3):749–764. doi: 10.1016/S0006-3495(78)85418-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whatley R., Ng S. K., Rogers J., McMurray W. C., Sanwal B. D. Developmental changes in gangliosides during myogenesis of a rat myoblast cell line and its drug resistant variants. Biochem Biophys Res Commun. 1976 May 3;70(1):180–185. doi: 10.1016/0006-291x(76)91125-6. [DOI] [PubMed] [Google Scholar]


