Abstract
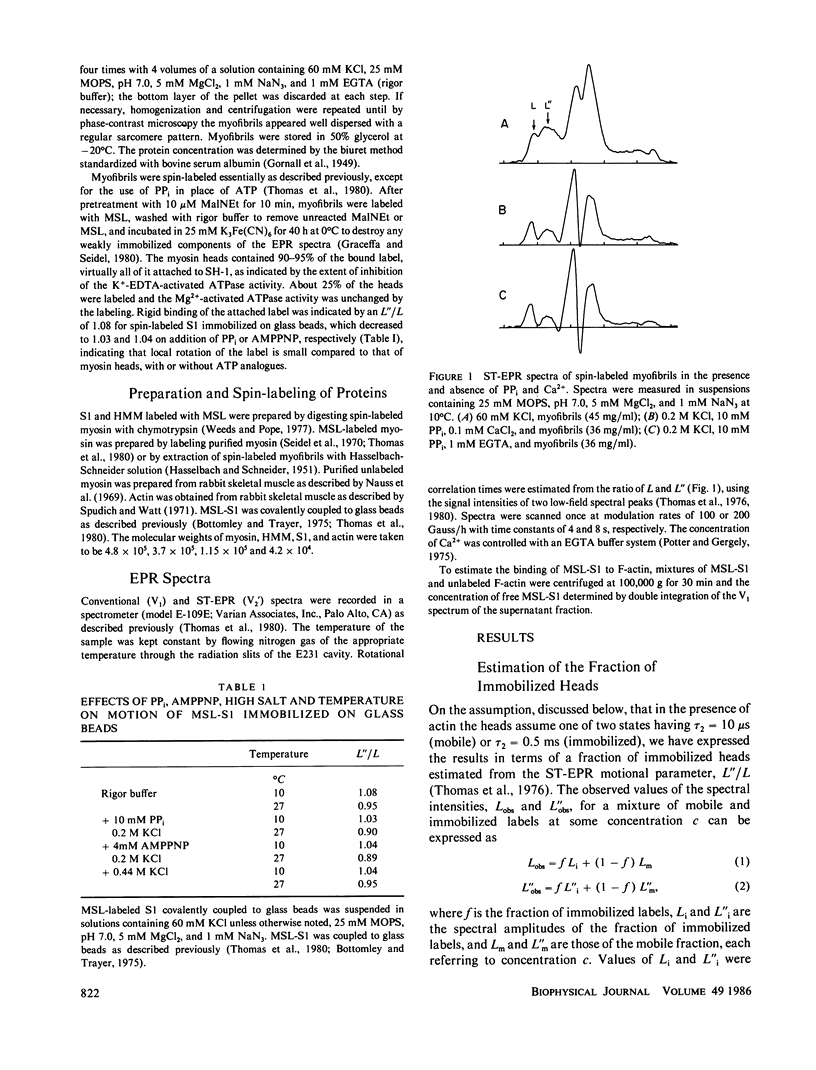
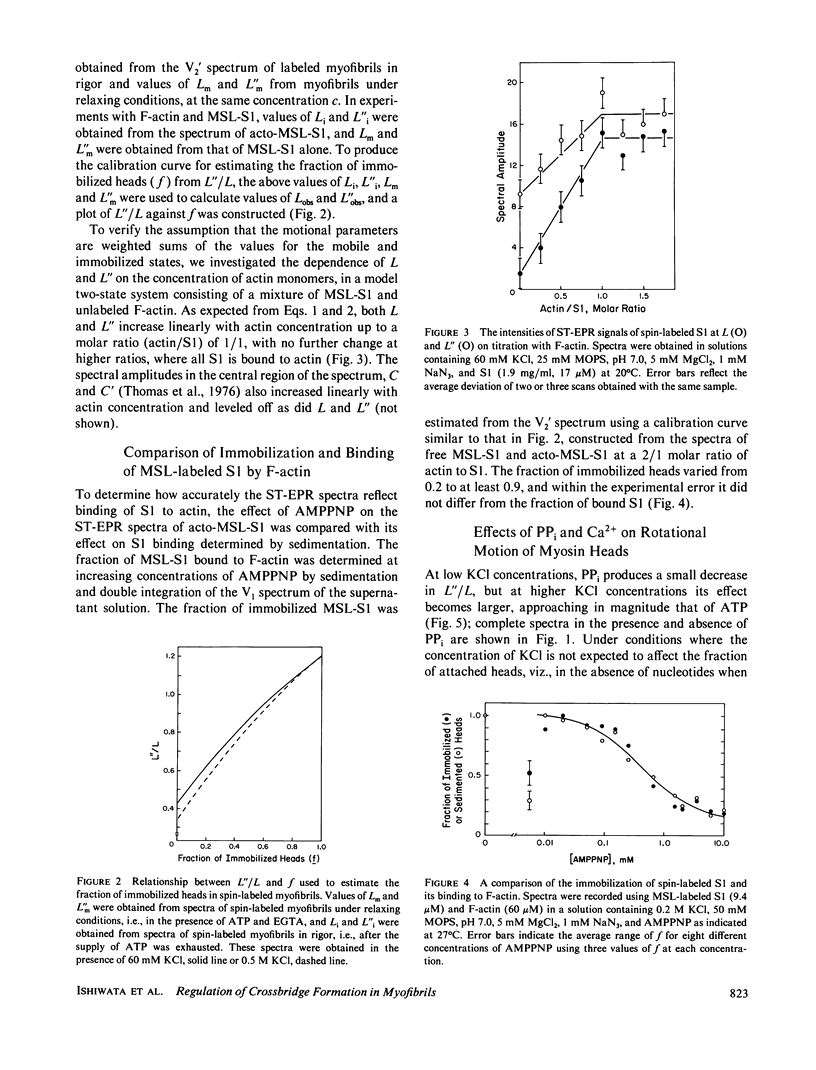
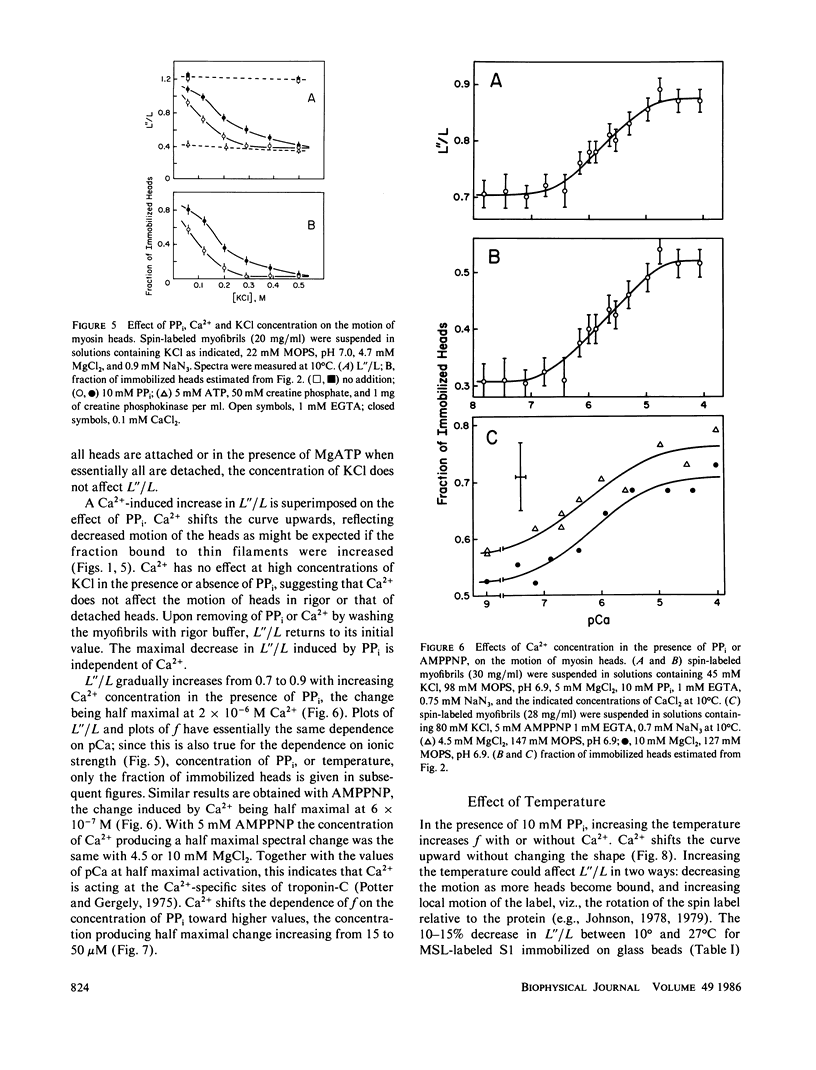
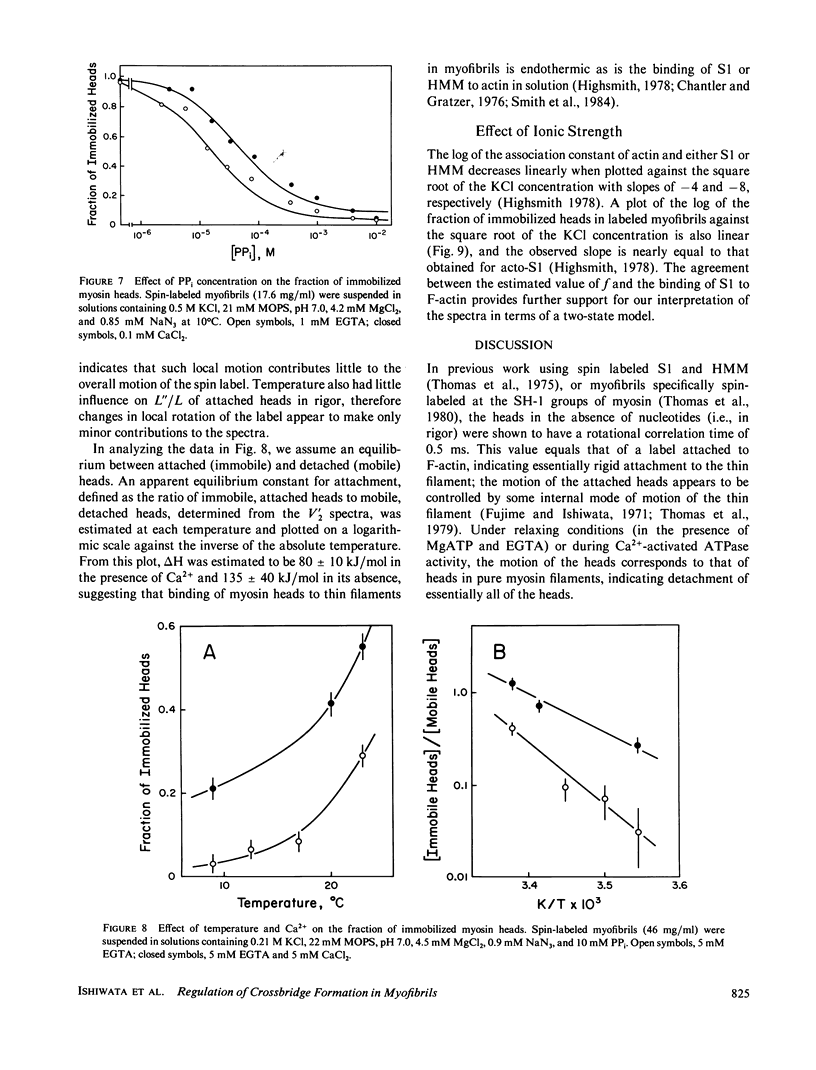
The rotational motion of rigidly spin-labeled myosin heads of glycerinated myofibrils as reflected in saturation-transfer EPR spectra behaves to a first approximation as though the heads consist of two populations with different rotational motions. An immobilized fraction has a correlation time (tau 2) of approximately 0.5 ms, comparable to that of spin-labeled subfragment-1 (S1) bound to thin filaments, while a mobile fraction has a tau 2 of 10 microseconds, comparable to that of the heads of purified myosin filaments. The effects of nonhydrolyzable ATP analogues, potassium pyrophosphate (PPi), or adenylyl imidodiphosphate, Ca2+, temperature, or ionic strength on the spectra can be analyzed in terms of the fraction of myosin heads immobilized by attachment to thin filaments, without requiring changes in the motion of either attached or detached heads.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bottomley R. C., Trayer I. P. Affinity chromatography of immobilized actin and myosin. Biochem J. 1975 Aug;149(2):365–379. doi: 10.1042/bj1490365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B., Schoenberg M., Chalovich J. M., Greene L. E., Eisenberg E. Evidence for cross-bridge attachment in relaxed muscle at low ionic strength. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7288–7291. doi: 10.1073/pnas.79.23.7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalovich J. M., Chock P. B., Eisenberg E. Mechanism of action of troponin . tropomyosin. Inhibition of actomyosin ATPase activity without inhibition of myosin binding to actin. J Biol Chem. 1981 Jan 25;256(2):575–578. [PMC free article] [PubMed] [Google Scholar]
- Chalovich J. M., Eisenberg E. Inhibition of actomyosin ATPase activity by troponin-tropomyosin without blocking the binding of myosin to actin. J Biol Chem. 1982 Mar 10;257(5):2432–2437. [PMC free article] [PubMed] [Google Scholar]
- Chantler P. D., Gratzer W. B. The interaction of actin monomers with myosin heads and other muscle proteins. Biochemistry. 1976 May 18;15(10):2219–2225. doi: 10.1021/bi00655a030. [DOI] [PubMed] [Google Scholar]
- Cooke R., Crowder M. S., Thomas D. D. Orientation of spin labels attached to cross-bridges in contracting muscle fibres. Nature. 1982 Dec 23;300(5894):776–778. doi: 10.1038/300776a0. [DOI] [PubMed] [Google Scholar]
- Cooke R. Stress does not alter the conformation of a domain of the myosin cross-bridge in rigor muscle fibres. Nature. 1981 Dec 10;294(5841):570–571. doi: 10.1038/294570a0. [DOI] [PubMed] [Google Scholar]
- Eads T. M., Thomas D. D., Austin R. H. Microsecond rotational motions of eosin-labeled myosin measured by time-resolved anisotropy of absorption and phosphorescence. J Mol Biol. 1984 Oct 15;179(1):55–81. doi: 10.1016/0022-2836(84)90306-1. [DOI] [PubMed] [Google Scholar]
- Fujime S., Ishiwata S. Dynamic study of F-actin by quasielastic scattering of laser light. J Mol Biol. 1971 Nov 28;62(1):251–265. doi: 10.1016/0022-2836(71)90144-6. [DOI] [PubMed] [Google Scholar]
- Graceffa P., Seidel J. C. A reaction involving protein sulfhydryl groups, a bound spin-label, and K3Fe(CN)6 as a probe of sulfhydryl proximity in myosin. Biochemistry. 1980 Jan 8;19(1):33–39. doi: 10.1021/bi00542a006. [DOI] [PubMed] [Google Scholar]
- Greene L. E., Eisenberg E. Cooperative binding of myosin subfragment-1 to the actin-troponin-tropomyosin complex. Proc Natl Acad Sci U S A. 1980 May;77(5):2616–2620. doi: 10.1073/pnas.77.5.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene L. E., Eisenberg E. Formation of a ternary complex: actin, 5'-adenylyl imidodiphosphate, and the subfragments of myosin. Proc Natl Acad Sci U S A. 1978 Jan;75(1):54–58. doi: 10.1073/pnas.75.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASSELBACH W., SCHNEIDER G. Der L-Myosin- und Aktingehalt des Kaninchenmuskels. Biochem Z. 1951;321(6):462–475. [PubMed] [Google Scholar]
- Highsmith S. Heavy meromyosin binds actin with negative cooperativity. Biochemistry. 1978 Jan 10;17(1):22–26. doi: 10.1021/bi00594a004. [DOI] [PubMed] [Google Scholar]
- Highsmith S. Interactions of the actin and nucleotide binding sites on myosin subfragment 1. J Biol Chem. 1976 Oct 25;251(20):6170–6172. [PubMed] [Google Scholar]
- Highsmith S., Mendelson R. A., Morales M. F. Affinity of myosin S-1 for F-actin, measured by time-resolved fluorescence anisotropy. Proc Natl Acad Sci U S A. 1976 Jan;73(1):133–137. doi: 10.1073/pnas.73.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highsmith S. The effects of temperature and salts on myosin subfragment-1 and F-actin association. Arch Biochem Biophys. 1977 Apr 30;180(2):404–408. doi: 10.1016/0003-9861(77)90054-6. [DOI] [PubMed] [Google Scholar]
- Huxley A. F., Simmons R. M. Proposed mechanism of force generation in striated muscle. Nature. 1971 Oct 22;233(5321):533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- Johnson M. E. Librational motion of an "immobilized" spin label: hemoglobin spin labeled by a maleimide derivative. Biochemistry. 1978 Apr 4;17(7):1223–1228. doi: 10.1021/bi00600a014. [DOI] [PubMed] [Google Scholar]
- Johnson M. E. Spin-label techniques for monitoring macromolecular rotational motion: empirical calibration under nonideal conditions. Biochemistry. 1979 Jan 23;18(2):378–384. doi: 10.1021/bi00569a023. [DOI] [PubMed] [Google Scholar]
- Mendelson R. A., Cheung P. Muscle crossbridges: absence of direct effect of calcium on movement away from the thick filaments. Science. 1976 Oct 8;194(4261):190–192. doi: 10.1126/science.194.4261.190-a. [DOI] [PubMed] [Google Scholar]
- Mendelson R. A., Morales M. F., Botts J. Segmental flexibility of the S-1 moiety of myosin. Biochemistry. 1973 Jun 5;12(12):2250–2255. doi: 10.1021/bi00736a011. [DOI] [PubMed] [Google Scholar]
- Mendelson R., Cheung P. H. Intrinsic segmental flexibility of the S-1 moiety of myosin using single-headed myosin. Biochemistry. 1978 May 30;17(11):2139–2148. doi: 10.1021/bi00604a018. [DOI] [PubMed] [Google Scholar]
- Nauss K. M., Kitagawa S., Gergely J. Pyrophosphate binding to and adenosine triphosphatase activity of myosin and its proteolytic fragments. Implications for the substructure of myosin. J Biol Chem. 1969 Feb 25;244(4):755–765. [PubMed] [Google Scholar]
- Potter J. D., Gergely J. The calcium and magnesium binding sites on troponin and their role in the regulation of myofibrillar adenosine triphosphatase. J Biol Chem. 1975 Jun 25;250(12):4628–4633. [PubMed] [Google Scholar]
- Seidel J. C., Chopek M., Gergely J. Effect of nucleotides and pyrophosphate on spin labels bound to S1 thiol groups of myosin. Biochemistry. 1970 Aug 4;9(16):3265–3272. doi: 10.1021/bi00818a021. [DOI] [PubMed] [Google Scholar]
- Smith S. J., White H. D., Woledge R. C. Microcalorimetric measurement of the enthalpy of binding of rabbit skeletal myosin subfragment 1 and heavy meromyosin to F-actin. J Biol Chem. 1984 Aug 25;259(16):10303–10308. [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Thomas D. D., Cooke R. Orientation of spin-labeled myosin heads in glycerinated muscle fibers. Biophys J. 1980 Dec;32(3):891–906. doi: 10.1016/S0006-3495(80)85024-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. D., Ishiwata S., Seidel J. C., Gergely J. Submillisecond rotational dynamics of spin-labeled myosin heads in myofibrils. Biophys J. 1980 Dec;32(3):873–889. doi: 10.1016/S0006-3495(80)85023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. D., Seidel J. C., Gergely J. Rotational dynamics of spin-labeled F-actin in the sub-millisecond time range. J Mol Biol. 1979 Aug 15;132(3):257–273. doi: 10.1016/0022-2836(79)90259-6. [DOI] [PubMed] [Google Scholar]
- Thomas D. D., Seidel J. C., Hyde J. S., Gergely J. Motion of subfragment-1 in myosin and its supramolecular complexes: saturation transfer electron paramagnetic resonance. Proc Natl Acad Sci U S A. 1975 May;72(5):1729–1733. doi: 10.1073/pnas.72.5.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner P. D., Giniger E. Calcium-sensitive binding of heavy meromyosin to regulated actin in the presence of ATP. J Biol Chem. 1981 Dec 25;256(24):12647–12650. [PubMed] [Google Scholar]
- Wagner P. D., Stone D. B. Calcium-sensitive binding of heavy meromyosin to regulated actin requires light chain 2 and the head-tail junction. Biochemistry. 1983 Mar 15;22(6):1334–1342. doi: 10.1021/bi00275a003. [DOI] [PubMed] [Google Scholar]
- Weeds A. G., Pope B. Studies on the chymotryptic digestion of myosin. Effects of divalent cations on proteolytic susceptibility. J Mol Biol. 1977 Apr;111(2):129–157. doi: 10.1016/s0022-2836(77)80119-8. [DOI] [PubMed] [Google Scholar]


