Abstract
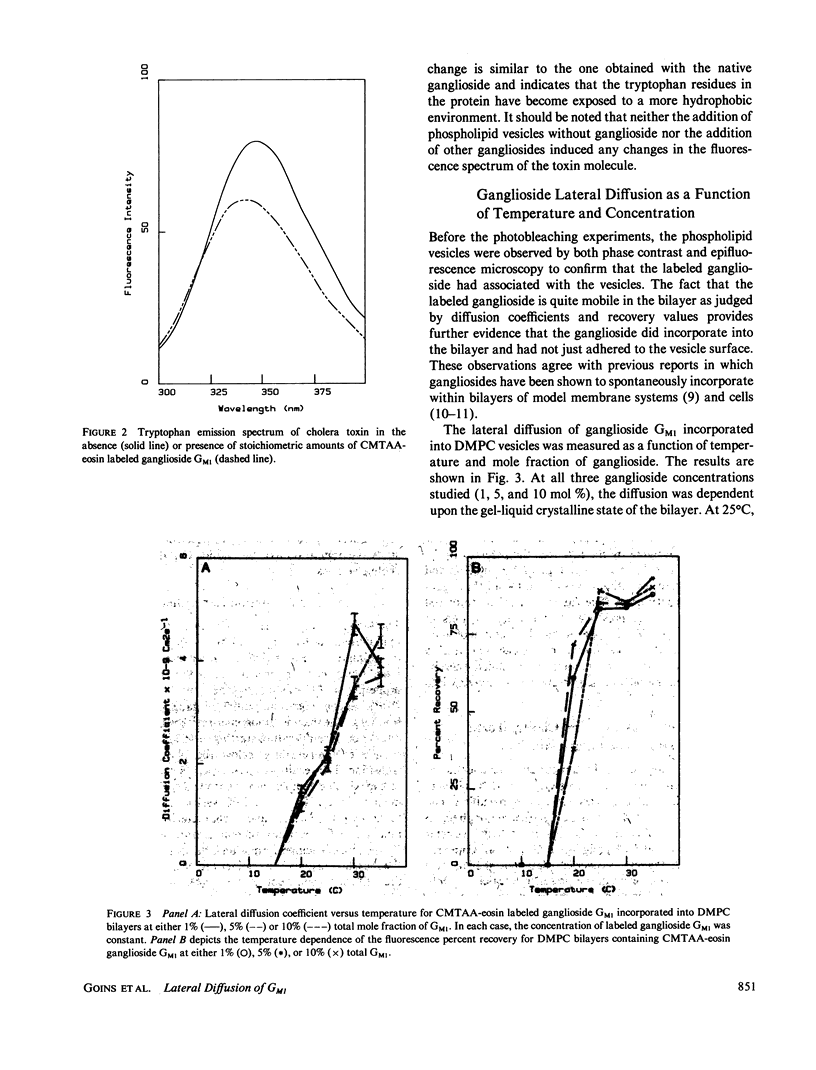
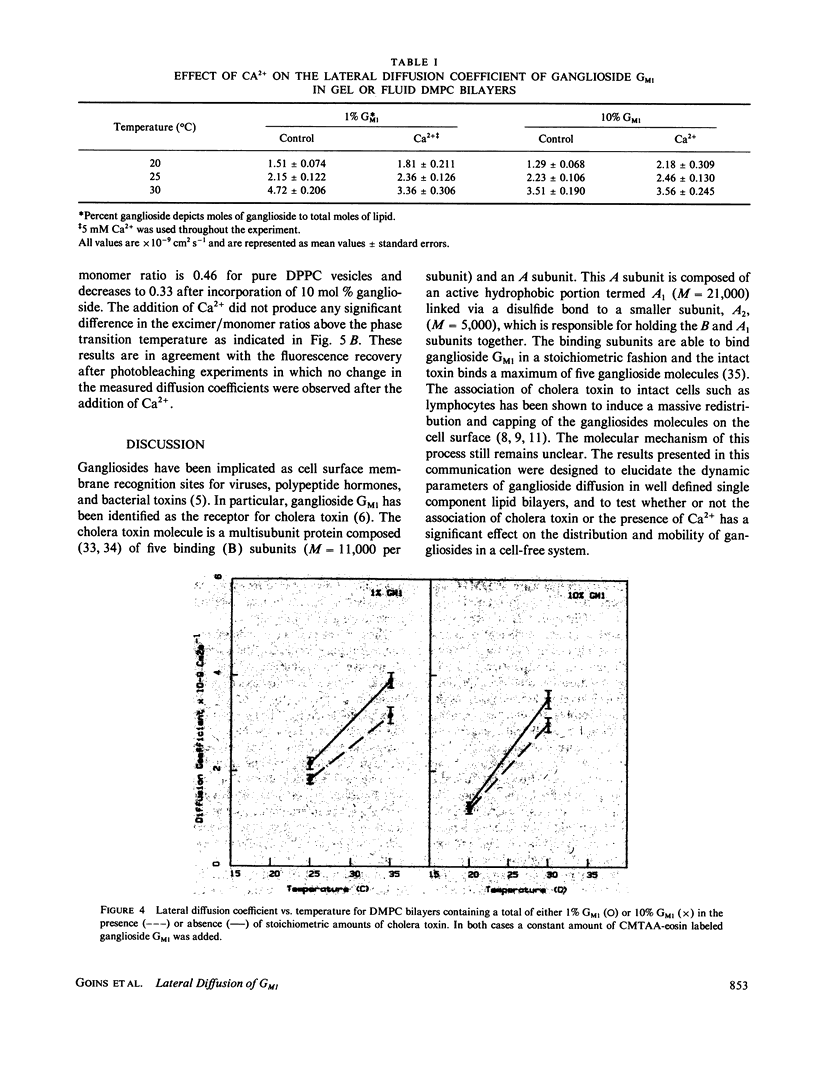
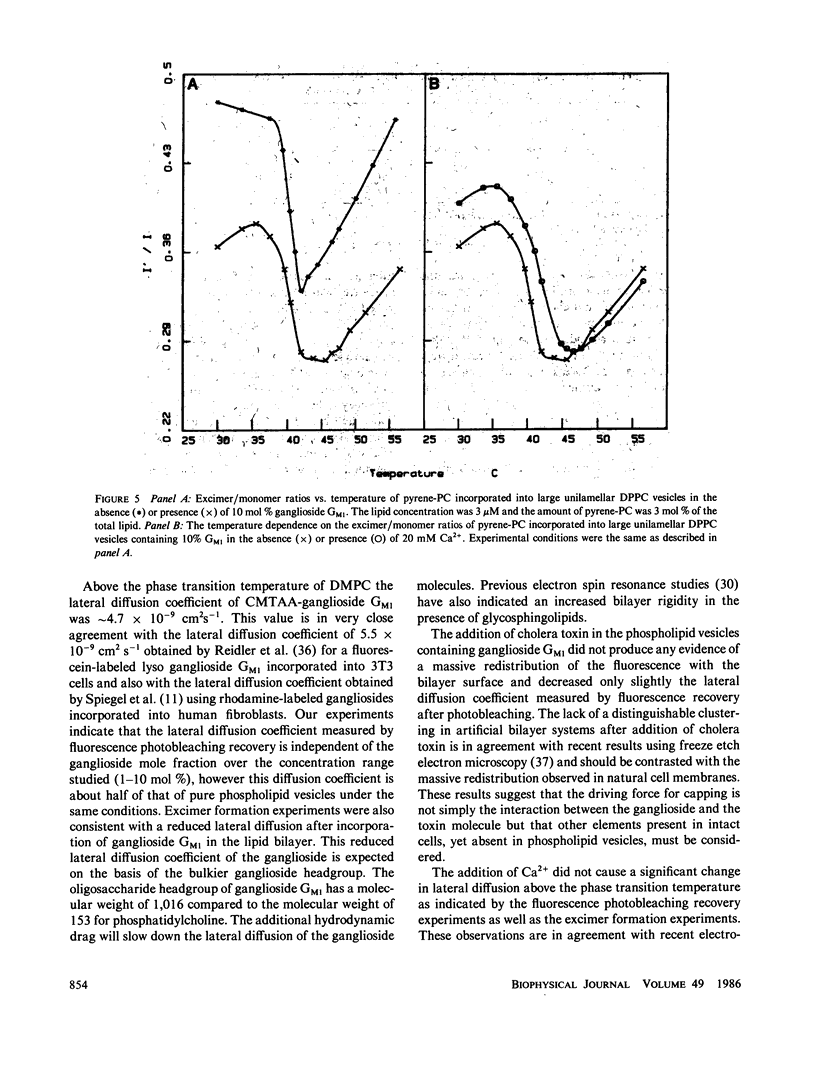
The lateral diffusion coefficient of ganglioside GM1 incorporated into preformed dimyristoylphosphatidylcholine (DMPC) vesicles has been investigated under a variety of conditions using the technique of fluorescence photobleaching recovery. For these studies the fluorescent probe 5-(((2-Carbohydrazino)methyl)thio)acetyl) amino eosin was covalently attached to the periodate-oxidized sialic acid residue of ganglioside GM1. This labeled ganglioside exhibited a behavior similar to that of the intact ganglioside, and was able to bind cholera toxin. The lateral diffusion coefficient of the ganglioside was dependent upon the gel-liquid crystalline transition of DMPC. Above Tm the lateral diffusion coefficient of the ganglioside was 4.7 X 10(-9) cm2 s-1 (with greater than 80% fluorescence recovery). This diffusion coefficient is significantly slower than the one previously observed for phospholipids in DMPC bilayers. The addition of increasing amounts of ganglioside, up to a maximum of 10 mol %, did not have a significant effect on the lateral diffusion coefficient or in the percent recovery. At 30 degrees C, the lateral mobility of ganglioside GM1 was not affected by the presence of 5 mM Ca2+, suggesting that, at least above Tm, Ca2+ does not induce a major perturbation in the lateral organization of the ganglioside molecules. The addition of stoichiometric amounts of cholera toxin to samples containing either 1 or 10 mol % ganglioside GM1 produced only a small decrease in the measured diffusion coefficient. The fluorescence recovery after photobleaching experiments were complemented with excimer formation experiments using pyrene-phosphatidylcholine. Above the transition temperature the presence of 10 mol % ganglioside GMI induced a large decrease in the rate of excimer formation. These results also indicated that the addition of ganglioside GMI to phospholipid bilayer vesicles induces a significant restriction in the lateral mobility parameters of the lipid bilayer and that the presence of Ca2' does not have a further effect in the mobility of the probe molecules.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barisas B. G., Leuther M. D. Fluorescence photobleaching recovery measurement of protein absolute diffusion constants. Biophys Chem. 1979 Sep;10(2):221–229. doi: 10.1016/0301-4622(79)85044-9. [DOI] [PubMed] [Google Scholar]
- Behr J. P., Lehn J. M. The binding of divalent cations by purified gangliosides. FEBS Lett. 1973 May 1;31(3):297–300. doi: 10.1016/0014-5793(73)80126-7. [DOI] [PubMed] [Google Scholar]
- Cherry R. J., Cogoli A., Oppliger M., Schneider G., Semenza G. A spectroscopic technique for measuring slow rotational diffusion of macromolecules. 1: Preparation and properties of a triplet probe. Biochemistry. 1976 Aug 24;15(17):3653–3656. doi: 10.1021/bi00662a001. [DOI] [PubMed] [Google Scholar]
- Cherry R. J., Nigg E. A., Beddard G. S. Oligosaccharide motion in erythrocyte membranes investigated by picosecond fluorescence polarization and microsecond dichroism of an optical probe. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5899–5903. doi: 10.1073/pnas.77.10.5899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey P. F., Webb W. W. Lateral diffusion in phospholipid bilayer membranes and multilamellar liquid crystals. Biochemistry. 1978 Jul 25;17(15):3046–3053. doi: 10.1021/bi00608a016. [DOI] [PubMed] [Google Scholar]
- Felgner P. L., Freire E., Barenholz Y., Thompson T. E. Asymmetric incorporation of trisialoganglioside into dipalmitoylphosphatidylcholine vesicles. Biochemistry. 1981 Apr 14;20(8):2168–2172. doi: 10.1021/bi00511a015. [DOI] [PubMed] [Google Scholar]
- Fishman P. H., Brady R. O. Biosynthesis and function of gangliosides. Science. 1976 Nov 26;194(4268):906–915. doi: 10.1126/science.185697. [DOI] [PubMed] [Google Scholar]
- Fishman P. H., Moss J., Osborne J. C., Jr Interaction of choleragen with the oligosaccharide of ganglioside GM1: evidence for multiple oligosaccharide binding sites. Biochemistry. 1978 Feb 21;17(4):711–716. doi: 10.1021/bi00597a024. [DOI] [PubMed] [Google Scholar]
- Fishman P. H. Role of membrane gangliosides in the binding and action of bacterial toxins. J Membr Biol. 1982;69(2):85–97. doi: 10.1007/BF01872268. [DOI] [PubMed] [Google Scholar]
- Galla H. J., Hartmann W. Excimer-forming lipids in membrane research. Chem Phys Lipids. 1980 Oct;27(3):199–219. doi: 10.1016/0009-3084(80)90036-5. [DOI] [PubMed] [Google Scholar]
- Galla H. J., Sackmann E. Lateral diffusion in the hydrophobic region of membranes: use of pyrene excimers as optical probes. Biochim Biophys Acta. 1974 Feb 26;339(1):103–115. doi: 10.1016/0005-2736(74)90336-8. [DOI] [PubMed] [Google Scholar]
- Gill D. M. The arrangement of subunits in cholera toxin. Biochemistry. 1976 Mar 23;15(6):1242–1248. doi: 10.1021/bi00651a011. [DOI] [PubMed] [Google Scholar]
- Goins B., Freire E. Lipid phase separations induced by the association of cholera toxin to phospholipid membranes containing ganglioside GM1. Biochemistry. 1985 Mar 26;24(7):1791–1797. doi: 10.1021/bi00328a033. [DOI] [PubMed] [Google Scholar]
- Hakomori S. Glycosphingolipids in cellular interaction, differentiation, and oncogenesis. Annu Rev Biochem. 1981;50:733–764. doi: 10.1146/annurev.bi.50.070181.003505. [DOI] [PubMed] [Google Scholar]
- Hansson H. A., Holmgren J., Svennerholm L. Ultrastructural localization of cell membrane GM1 ganglioside by cholera toxin. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3782–3786. doi: 10.1073/pnas.74.9.3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaques L. W., Brown E. B., Barrett J. M., Brey WS Jr Weltner W., Jr Sialic acid. A calcium-binding carbohydrate. J Biol Chem. 1977 Jul 10;252(13):4533–4538. [PubMed] [Google Scholar]
- Kundu S. K. Thin-layer chromatography of neutral glycosphingolipids and gangliosides. Methods Enzymol. 1981;72:185–204. doi: 10.1016/s0076-6879(81)72012-3. [DOI] [PubMed] [Google Scholar]
- Lai C. Y. The chemistry and biology of cholera toxin. CRC Crit Rev Biochem. 1980;9(3):171–206. doi: 10.3109/10409238009105434. [DOI] [PubMed] [Google Scholar]
- Ledeen R. W. Ganglioside structures and distribution: are they localized at the nerve ending? J Supramol Struct. 1978;8(1):1–17. doi: 10.1002/jss.400080102. [DOI] [PubMed] [Google Scholar]
- Leuther M. D., Peacock J. S., Krakauer H., Barisas B. G. Changes in lectin receptor lateral mobilities accompany lymphocyte stimulation. J Immunol. 1981 Sep;127(3):893–899. [PubMed] [Google Scholar]
- Mullin B. R., Aloj S. M., Fishman P. H., Lee G., Kohn L. D., Brady R. O. Cholera toxin interactions with thyrotropin receptors on thyroid plasma membranes. Proc Natl Acad Sci U S A. 1976 May;73(5):1679–1683. doi: 10.1073/pnas.73.5.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers M., Wortman C., Freire E. Modulation of neuraminidase activity by the physical state of phospholipid bilayers containing gangliosides Gd1a and Gt1b. Biochemistry. 1984 Mar 27;23(7):1442–1448. doi: 10.1021/bi00302a016. [DOI] [PubMed] [Google Scholar]
- Peacock J. S., Barisas B. G. Photobleaching recovery studies of T-independent antigen mobility on antibody-bearing liposomes. J Immunol. 1983 Dec;131(6):2924–2929. [PubMed] [Google Scholar]
- Rahmann H., Probst W., Mühleisen M. Gangliosides and synaptic transmission. Jpn J Exp Med. 1982 Dec;52(6):275–286. [PubMed] [Google Scholar]
- Révész T., Greaves M. Ligand-induced redistribution of lymphocyte membrane ganglioside GM1. Nature. 1975 Sep 11;257(5522):103–106. doi: 10.1038/257103a0. [DOI] [PubMed] [Google Scholar]
- Sedlacek H. H., Stärk J., Seiler F. R., Ziegler W., Wiegandt H. Cholera toxin induced redistribution of sialoglycolipid receptor at the lymphocyte membrane. FEBS Lett. 1976 Jan 15;61(2):272–276. doi: 10.1016/0014-5793(76)81055-1. [DOI] [PubMed] [Google Scholar]
- Sharom F. J., Grant C. W. A model for ganglioside behaviour in cell membranes. Biochim Biophys Acta. 1978 Feb 21;507(2):280–293. doi: 10.1016/0005-2736(78)90423-6. [DOI] [PubMed] [Google Scholar]
- Spiegel S., Kassis S., Wilchek M., Fishman P. H. Direct visualization of redistribution and capping of fluorescent gangliosides on lymphocytes. J Cell Biol. 1984 Nov;99(5):1575–1581. doi: 10.1083/jcb.99.5.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel S., Schlessinger J., Fishman P. H. Incorporation of fluorescent gangliosides into human fibroblasts: mobility, fate, and interaction with fibronectin. J Cell Biol. 1984 Aug;99(2):699–704. doi: 10.1083/jcb.99.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steck T. L., Dawson G. Topographical distribution of complex carbohydrates in the erythrocyte membrane. J Biol Chem. 1974 Apr 10;249(7):2135–2142. [PubMed] [Google Scholar]
- Thompson T. E., Allietta M., Brown R. E., Johnson M. L., Tillack T. W. Organization of ganglioside GM1 in phosphatidylcholine bilayers. Biochim Biophys Acta. 1985 Jul 25;817(2):229–237. doi: 10.1016/0005-2736(85)90024-0. [DOI] [PubMed] [Google Scholar]
- Tuszynski G. P., Knight L., Piperno J. R., Walsh P. N. A rapid method for removal of [125I]iodide following iodination of protein solutions. Anal Biochem. 1980 Jul 15;106(1):118–122. doi: 10.1016/0003-2697(80)90126-8. [DOI] [PubMed] [Google Scholar]


