Abstract
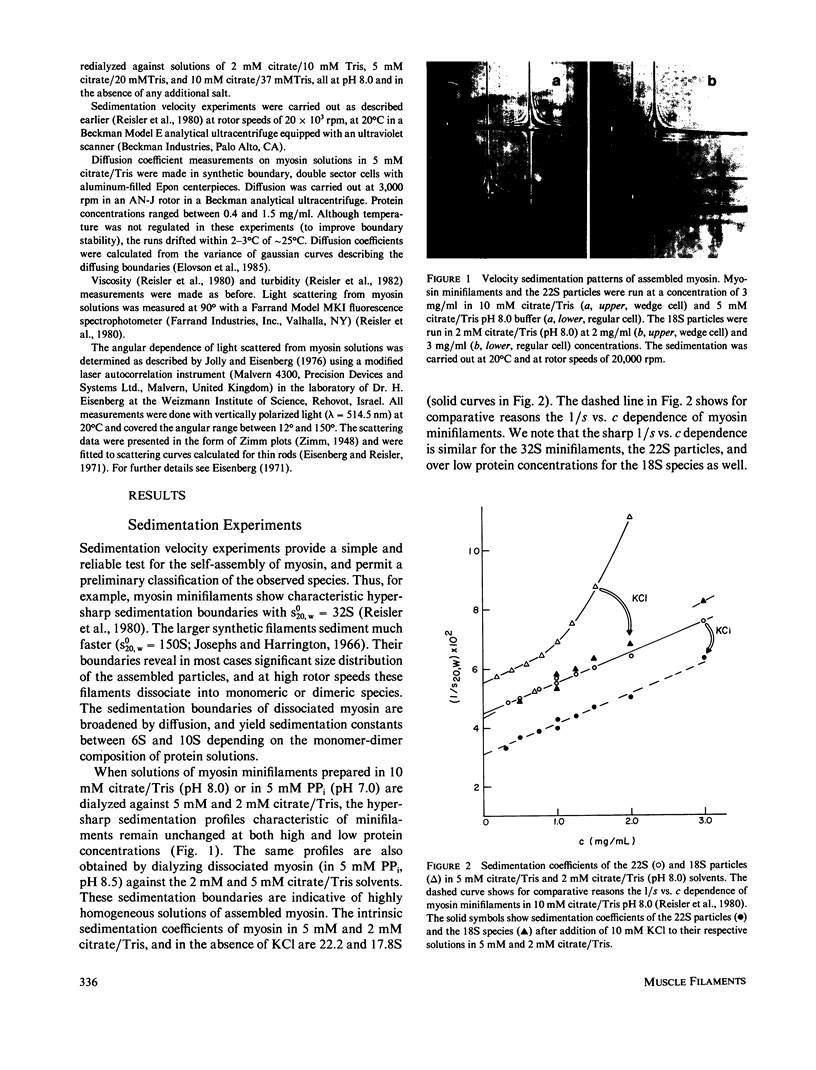
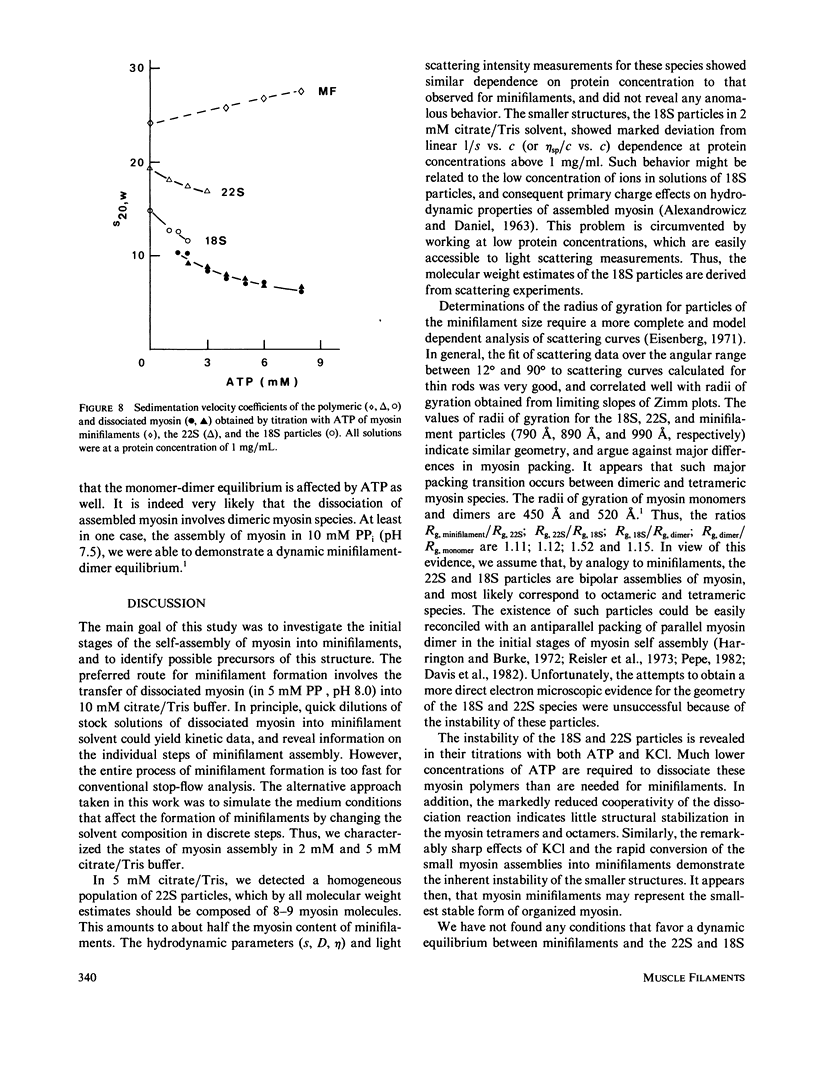
The self-assembly of myosin into filamentous structures is a highly cooperative and rapid process. Nevertheless, the presence of nonequivalent bonding interactions within the filament permits differential stabilization of several macromolecular assemblies of myosin under well-controlled ionic conditions in citrate/Tris buffer at pH 8.0. We have detected and characterized bipolar myosin minifilaments, myosin octamers, and tetramers by using light scattering, analytical ultracentrifugation, and viscosity techniques. These structures have molecular weights of 8.0 X 10(6), 3.9 X 10(6) g/mol, sedimentation coefficients of 32S, 22S, and 18S, and radii of gyration of 990 A, 890 A and 790, A, respectively. The similar radii of gyration indicate similar bipolar geometry for all these particles. The 32S minifilaments in 10 mM citrate/Tris buffer (pH 8.0) are the most stable species. The smaller 18S and 22S assemblies in 2 mM and 5 mM citrate/Tris, pH 8.0, are readily affected by low concentrations of KCl and fuse into the minifilament particles. The instability of the 18S and 22S forms of myosin assembly is also revealed by their titration with ATP. These structures are dissociated at lower ATP concentrations than the minifilaments and do not show the cooperative dissociation transitions characteristic of filaments and minifilaments. Sedimentation velocity analysis of the 18S and 22S species in the presence of ATP reveals the involvement of 10S myosin dimer in the dissociation of assembled myosin. The different forms of assembled myosin are discussed in the context of formation of myosin minifilaments.
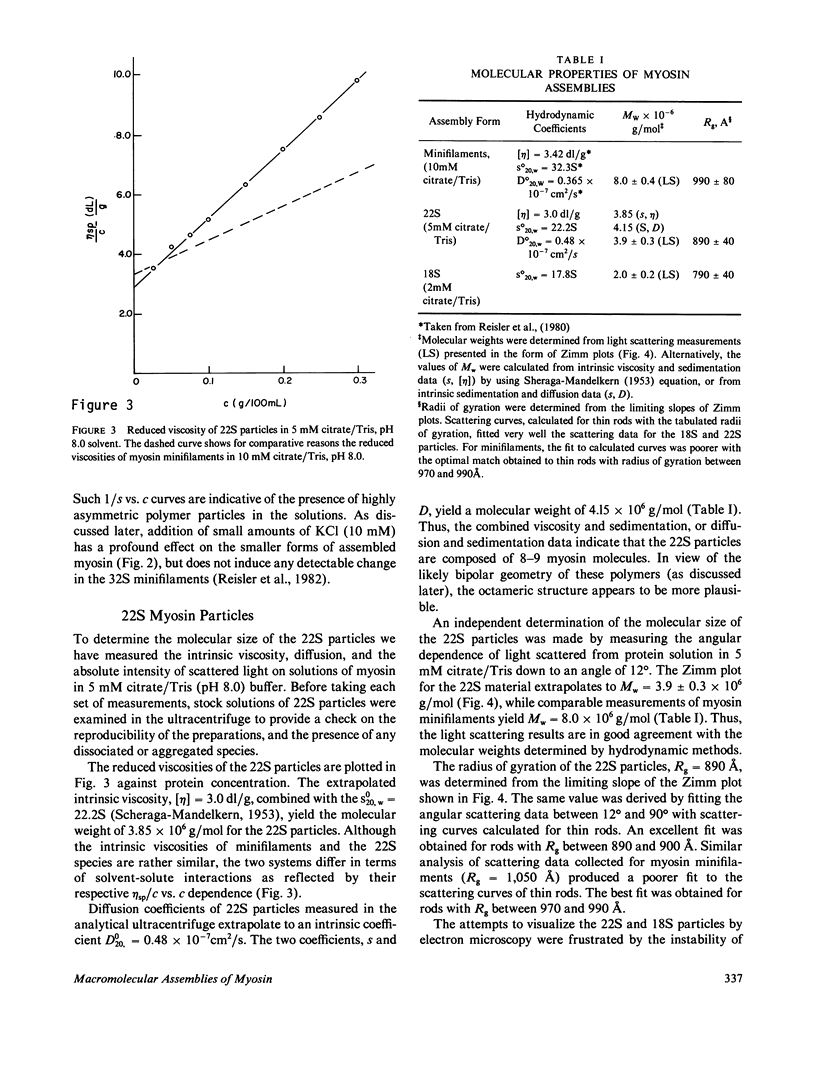
Full text
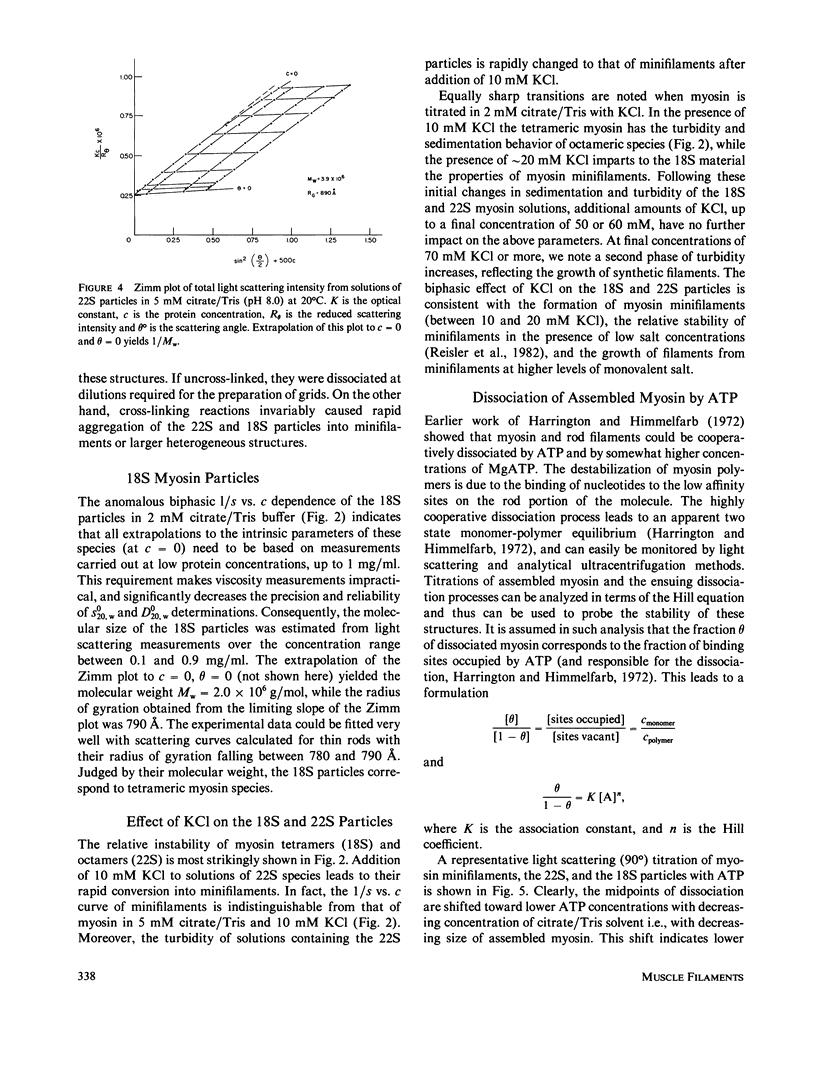
PDF
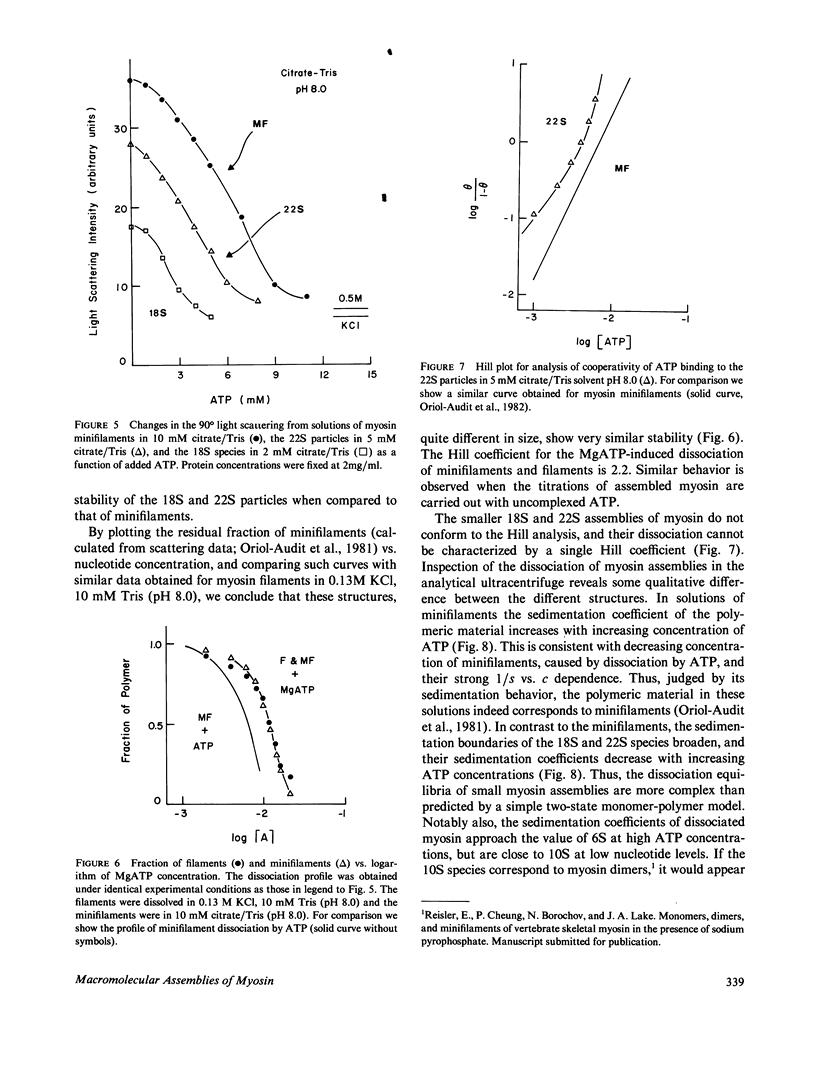

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Davis J. S., Buck J., Greene E. P. The myosin dimer: an intermediate in the self-assembly of the thick filament of vertebrate skeletal muscle. FEBS Lett. 1982 Apr 19;140(2):293–297. doi: 10.1016/0014-5793(82)80917-4. [DOI] [PubMed] [Google Scholar]
- Davis J. S. Pressure-jump studies on the length-regulation kinetics of the self-assembly of myosin from vertebrate skeletal muscle into thick filament. Biochem J. 1981 Aug 1;197(2):309–314. doi: 10.1042/bj1970309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. S. The influence of pressure on the self-assembly of the thick filament from the myosin of vertebrate skeletal muscle. Biochem J. 1981 Aug 1;197(2):301–308. doi: 10.1042/bj1970301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg H., Reisler E. Angular dependence of scattered light, rotary frictional coefficients, and distribution of sizes of associated oligomers in solutions of bovine liver glutamate dehydrogenase. Biopolymers. 1971;10(12):2363–2376. doi: 10.1002/bip.360101202. [DOI] [PubMed] [Google Scholar]
- Elovson J., Jacobs J. C., Schumaker V. N., Puppione D. L. Molecular weights of apoprotein B obtained from human low-density lipoprotein (apoprotein B-PI) and from rat very low density lipoprotein (apoprotein B-PIII). Biochemistry. 1985 Mar 12;24(6):1569–1578. doi: 10.1021/bi00327a042. [DOI] [PubMed] [Google Scholar]
- Godfrey J. E., Harrington W. F. Self-association in the myosin system at high ionic strength. I. Sensitivity of the interaction to pH and ionic environment. Biochemistry. 1970 Feb 17;9(4):886–893. doi: 10.1021/bi00806a025. [DOI] [PubMed] [Google Scholar]
- Harrington W. F., Burke M. Geometry of the myosin dimer in high-salt media. I. Association behavior of rod segments from myosin. Biochemistry. 1972 Apr 11;11(8):1448–1455. doi: 10.1021/bi00758a019. [DOI] [PubMed] [Google Scholar]
- Harrington W. F., Himmelfarb S. Effect of adenosine di- and triphosphates on the stability of synthetic myosin filaments. Biochemistry. 1972 Aug 1;11(16):2945–2952. doi: 10.1021/bi00766a004. [DOI] [PubMed] [Google Scholar]
- Harrington W. F., Rodgers M. E. Myosin. Annu Rev Biochem. 1984;53:35–73. doi: 10.1146/annurev.bi.53.070184.000343. [DOI] [PubMed] [Google Scholar]
- Higuchi H., Ishiwata S. Disassembly kinetics of thick filaments in rabbit skeletal muscle fibers. Effects of ionic strength, Ca2+ concentration, pH, temperature, and cross-bridges on the stability of thick filament structure. Biophys J. 1985 Mar;47(3):267–275. doi: 10.1016/S0006-3495(85)83916-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiwata S. Melting from both ends of an A-band in a myofibril. Observation with a phase-contrast microscope. J Biochem. 1981 May;89(5):1647–1650. doi: 10.1093/oxfordjournals.jbchem.a133361. [DOI] [PubMed] [Google Scholar]
- Jolly D., Eisenberg H. Photon correlation spectroscopy, total intensity light scattering with laser radiation, and hydrodynamic studies of a well fractionated DNA sample. Biopolymers. 1976 Jan;15(1):61–95. doi: 10.1002/bip.1976.360150107. [DOI] [PubMed] [Google Scholar]
- Josephs R., Harrington W. F. Studies on the formation and physical chemical properties of synthetic myosin filaments. Biochemistry. 1966 Nov;5(11):3474–3487. doi: 10.1021/bi00875a013. [DOI] [PubMed] [Google Scholar]
- Maw M. C., Rowe A. J. Fraying of A-filaments into three subfilaments. Nature. 1980 Jul 24;286(5771):412–414. doi: 10.1038/286412a0. [DOI] [PubMed] [Google Scholar]
- McLachlan A. D., Karn J. Periodic charge distributions in the myosin rod amino acid sequence match cross-bridge spacings in muscle. Nature. 1982 Sep 16;299(5880):226–231. doi: 10.1038/299226a0. [DOI] [PubMed] [Google Scholar]
- McLachlan A. D., Karn J. Periodic features in the amino acid sequence of nematode myosin rod. J Mol Biol. 1983 Mar 15;164(4):605–626. doi: 10.1016/0022-2836(83)90053-0. [DOI] [PubMed] [Google Scholar]
- Niederman R., Peters L. K. Native bare zone assemblage nucleates myosin filament assembly. J Mol Biol. 1982 Nov 15;161(4):505–517. doi: 10.1016/0022-2836(82)90404-1. [DOI] [PubMed] [Google Scholar]
- Oriol-Audit C., Lake J. A., Reisler E. Structural changes in synthetic myosin minifilaments and their dissociation by adenosine triphosphate and pyrophosphate. Biochemistry. 1981 Feb 17;20(4):679–686. doi: 10.1021/bi00507a002. [DOI] [PubMed] [Google Scholar]
- Reisler E., Burke M., Josephs R., Harrington W. F. Crosslinking of myosin and myosin filaments. J Mechanochem Cell Motil. 1973;2(3):163–179. [PubMed] [Google Scholar]
- Reisler E., Cheung P., Oriol-Audit C., Lake J. A. Growth of synthetic myosin filaments from myosin minifilaments. Biochemistry. 1982 Feb 16;21(4):701–707. doi: 10.1021/bi00533a018. [DOI] [PubMed] [Google Scholar]
- Reisler E., Smith C., Seegan G. Myosin minifilaments. J Mol Biol. 1980 Oct 15;143(1):129–145. doi: 10.1016/0022-2836(80)90127-8. [DOI] [PubMed] [Google Scholar]
- Trinick J., Cooper J. Sequential disassembly of vertebrate muscle thick filaments. J Mol Biol. 1980 Aug 15;141(3):315–321. doi: 10.1016/0022-2836(80)90183-7. [DOI] [PubMed] [Google Scholar]



