Abstract
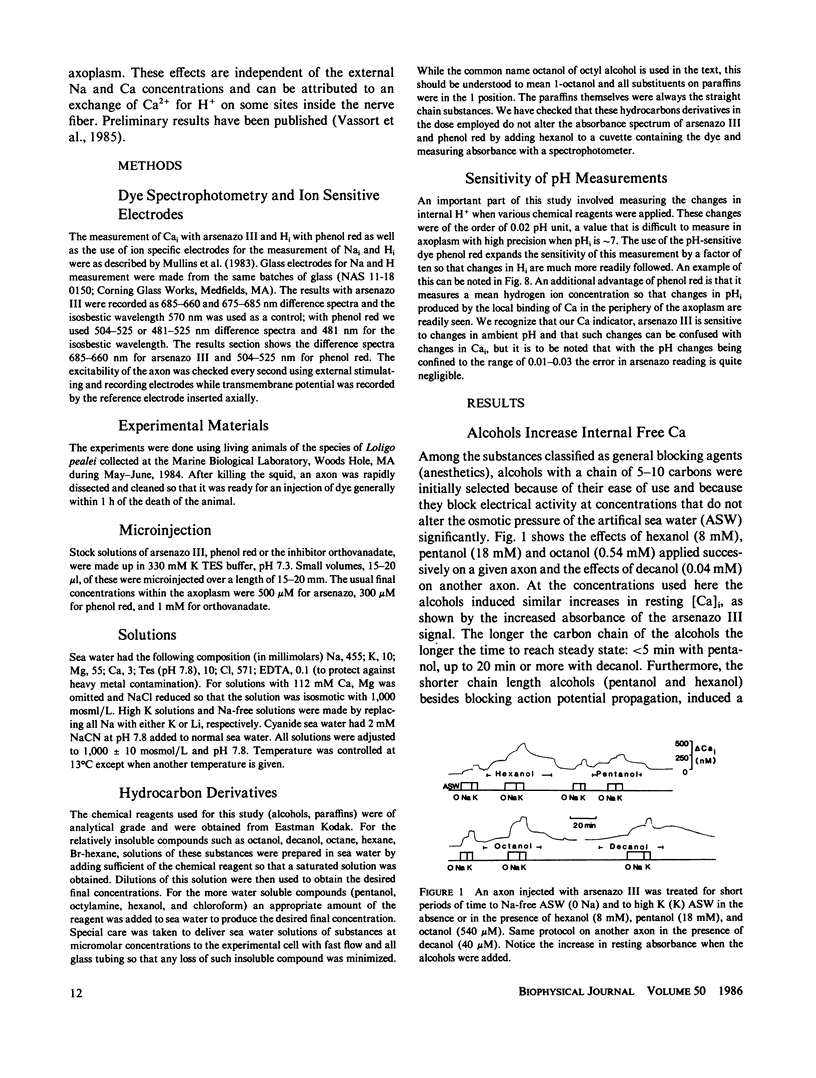
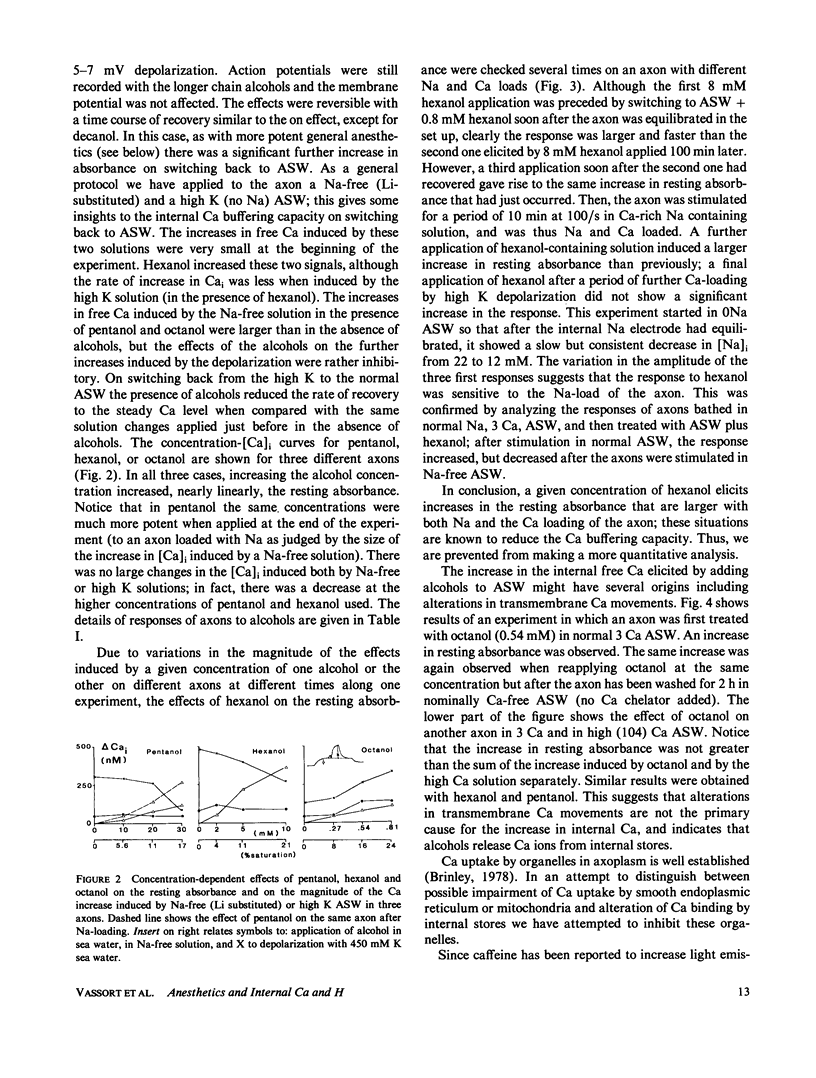
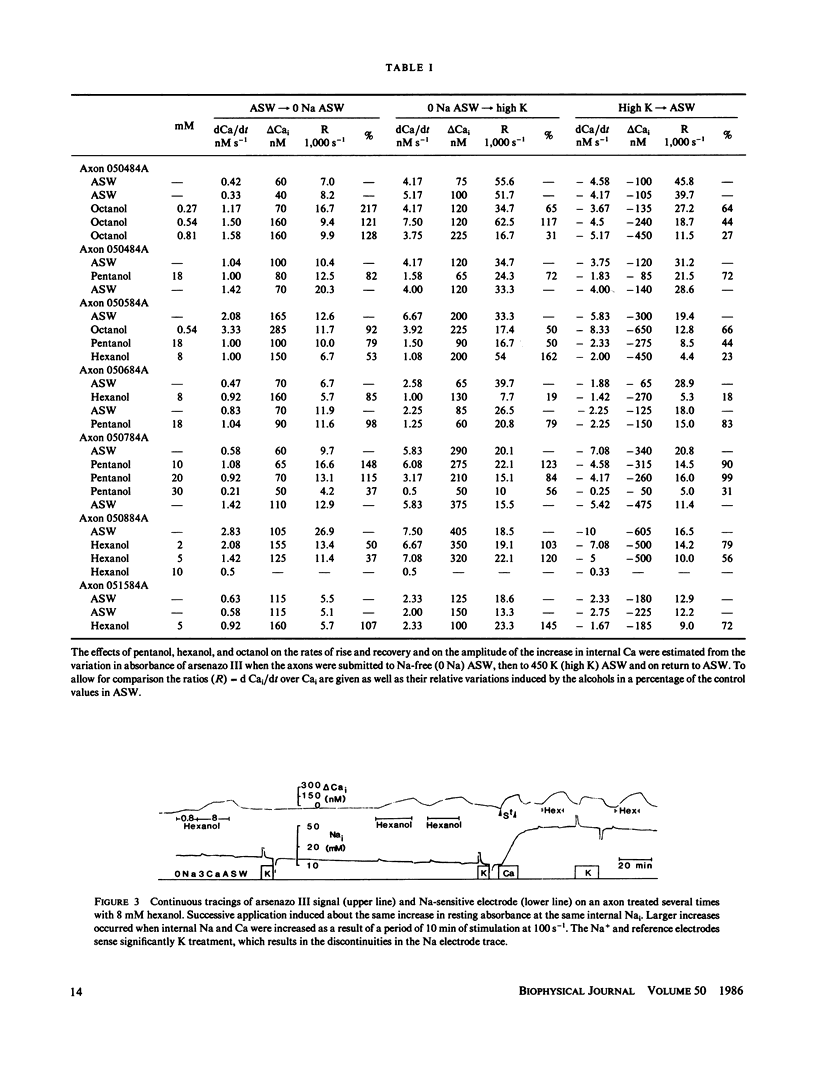
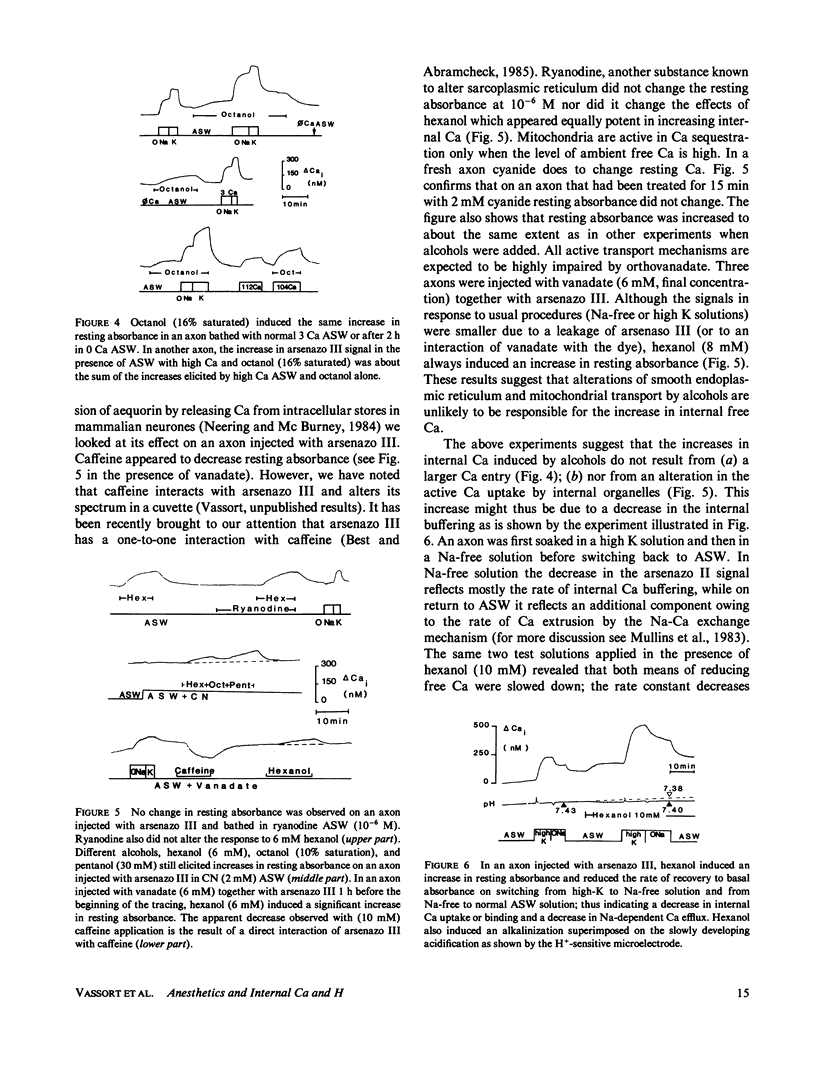
Squid axons were injected with arsenazo III and treated with sea water containing compounds usually classified as general anesthetics, (pentanol-decanol and a variety of hydrocarbons and their derivatives). Such treatment led to an increase in absorbance by arsenazo III at wavelengths sensitive to [Ca]i. The effect was independent of the presence or absence of Ca++ in sea water and it was not modified by substances that release Ca from internal stores. The effect was easily reversible. In axons injected with phenol red or impaled with a glass electrode sensitive to H+, a similar treatment led to an alkalinization that was also readily reversible. Both Ca release and the change to an alkaline pH had identical time courses. The dose required for action by all of the chemical agents studied could be predicted from a knowledge of their fractional saturation in sea water, i.e. from their thermodynamic activity. For compounds with 8-10 carbon atoms, Ca-release effects can occur at concentration less than those necessary to block either conduction or Na/Ca exchange. A special chemical agent was octylamine, which induced a marked rise in pHi and in addition its nonionic form produced the typical Ca release associated with general anesthetics.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. F., Schapira A. H. Anaesthetics increase light emission from aequorin at constant ionised calcium. Nature. 1980 Mar 13;284(5752):168–169. doi: 10.1038/284168a0. [DOI] [PubMed] [Google Scholar]
- Best P. M., Abramcheck C. W. The metallochromic indicator dye Arsenazo III forms 1:1 complexes with caffeine. Biochim Biophys Acta. 1985 Jan 28;838(1):179–182. doi: 10.1016/0304-4165(85)90265-x. [DOI] [PubMed] [Google Scholar]
- Boron W. F., De Weer P. Intracellular pH transients in squid giant axons caused by CO2, NH3, and metabolic inhibitors. J Gen Physiol. 1976 Jan;67(1):91–112. doi: 10.1085/jgp.67.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boron W. F., Russell J. M. Stoichiometry and ion dependencies of the intracellular-pH-regulating mechanism in squid giant axons. J Gen Physiol. 1983 Mar;81(3):373–399. doi: 10.1085/jgp.81.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinley F. J., Jr Calcium buffering in squid axons. Annu Rev Biophys Bioeng. 1978;7:363–392. doi: 10.1146/annurev.bb.07.060178.002051. [DOI] [PubMed] [Google Scholar]
- Brinley F. J., Jr, Tiffert T., Scarpa A., Mullins L. J. Intracellular calcium buffering capacity in isolated squid axons. J Gen Physiol. 1977 Sep;70(3):355–384. doi: 10.1085/jgp.70.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlen P. L., Gurevich N., Durand D. Ethanol in low doses augments calcium-mediated mechanisms measured intracellularly in hippocampal neurons. Science. 1982 Jan 15;215(4530):306–309. doi: 10.1126/science.7053581. [DOI] [PubMed] [Google Scholar]
- Hauser H., Dawson R. M. The displacement of calcium ions from phospholipid monolayers by pharmacologically active and other organic bases. Biochem J. 1968 Oct;109(5):909–916. doi: 10.1042/bj1090909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon D. A., Hendry B. M., Levinson S. R., Requena J. Anaesthesia by the n-alkanes. A comparative study of nerve impulse blockage and the properties of black lipid bilayer membranes. Biochim Biophys Acta. 1977 Oct 3;470(1):17–34. doi: 10.1016/0005-2736(77)90058-x. [DOI] [PubMed] [Google Scholar]
- Haydon D. A., Urban B. W. The action of alcohols and other non-ionic surface active substances on the sodium current of the squid giant axon. J Physiol. 1983 Aug;341:411–427. doi: 10.1113/jphysiol.1983.sp014813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon D. A., Urban B. W. The action of hydrocarbons and carbon tetrachloride on the sodium current of the squid giant axon. J Physiol. 1983 May;338:435–450. doi: 10.1113/jphysiol.1983.sp014682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon D. A., Urban B. W. The effects of some inhalation anaesthetics on the sodium current of the squid giant axon. J Physiol. 1983 Aug;341:429–439. doi: 10.1113/jphysiol.1983.sp014814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low P. S., Lloyd D. H., Stein T. M., Rogers J. A., 3rd Calcium displacement by local anesthetics. Dependence on pH and anesthetic charge. J Biol Chem. 1979 May 25;254(10):4119–4125. [PubMed] [Google Scholar]
- Michaelis M. L., Michaelis E. K. Alcohol and local anesthetic effects on Na+-dependent Ca2+ fluxes in brain synaptic membrane vesicles. Biochem Pharmacol. 1983 Mar 15;32(6):963–969. doi: 10.1016/0006-2952(83)90612-3. [DOI] [PubMed] [Google Scholar]
- Mullins L. J., Tiffert T., Vassort G., Whittembury J. Effects of internal sodium and hydrogen ions and of external calcium ions and membrane potential on calcium entry in squid axons. J Physiol. 1983 May;338:295–319. doi: 10.1113/jphysiol.1983.sp014674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neering I. R., McBurney R. N. Role for microsomal Ca storage in mammalian neurones? Nature. 1984 May 10;309(5964):158–160. doi: 10.1038/309158a0. [DOI] [PubMed] [Google Scholar]
- Requena J., Mullins L. J., Brinley F. J., Jr Calcium content and net fluxes in squid giant axons. J Gen Physiol. 1979 Mar;73(3):327–342. doi: 10.1085/jgp.73.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Requena J., Whittembury J., Tiffert T., Eisner D. A., Mullins L. J. The influence of chemical agents on the level of ionized [Ca2+] in squid axons. J Gen Physiol. 1985 Jun;85(6):789–804. doi: 10.1085/jgp.85.6.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross D. H. Selective action of alcohols on cerebral calcium levels. Ann N Y Acad Sci. 1976;273:280–294. doi: 10.1111/j.1749-6632.1976.tb52891.x. [DOI] [PubMed] [Google Scholar]
- Salama G., Scarpa A. Enhanced Ca2+ uptake and ATPase activity of sarcoplasmic reticulum in the presence of diethyl ether. J Biol Chem. 1980 Jul 25;255(14):6525–6528. [PubMed] [Google Scholar]
- Seeman P., Chau M., Goldberg M., Sauks T., Sax L. The binding of Ca2+ to the cell membrane increased by volatile anesthetics (alcohols, acetone, ether) which induce sensitization of nerve or muscle. Biochim Biophys Acta. 1971 Feb 2;225(2):185–193. doi: 10.1016/0005-2736(71)90211-2. [DOI] [PubMed] [Google Scholar]
- Yingst D. R., Polasek P. M., Kilgore P. The effect of ethanol on the passive Ca permeability of human red cell ghosts measured by means of arsenazo III. Biochim Biophys Acta. 1985 Mar 14;813(2):277–281. doi: 10.1016/0005-2736(85)90242-1. [DOI] [PubMed] [Google Scholar]


