Abstract
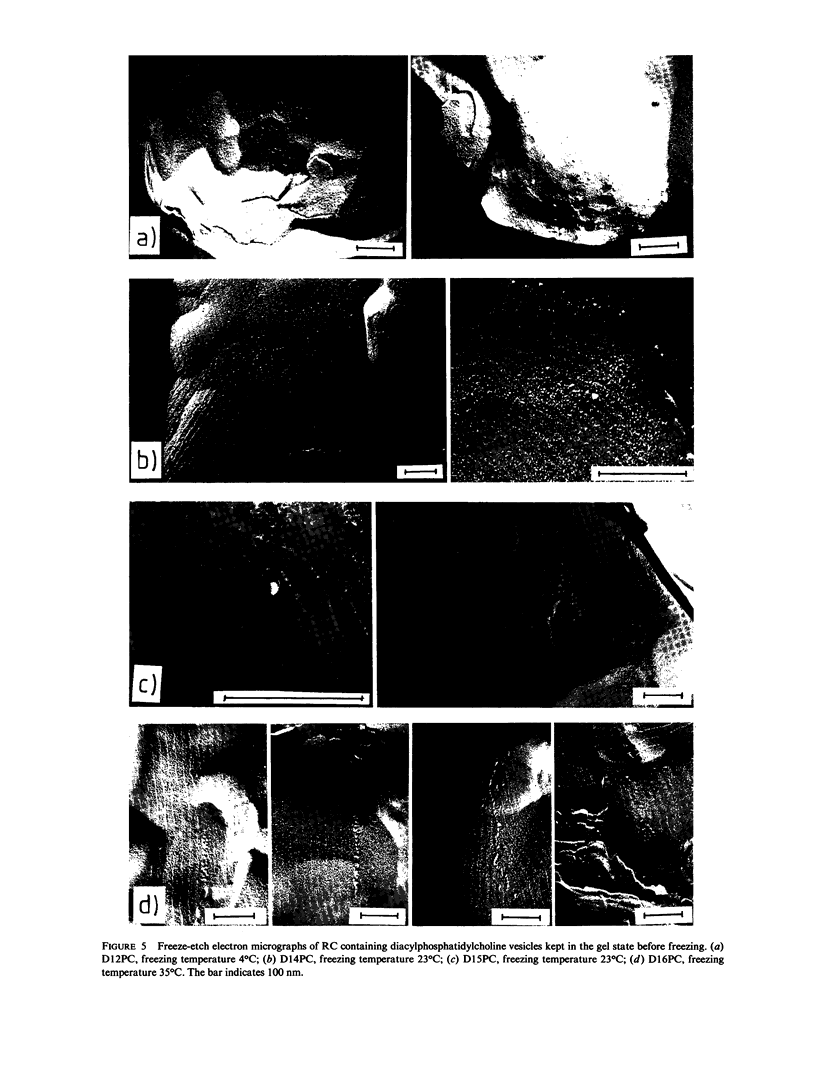
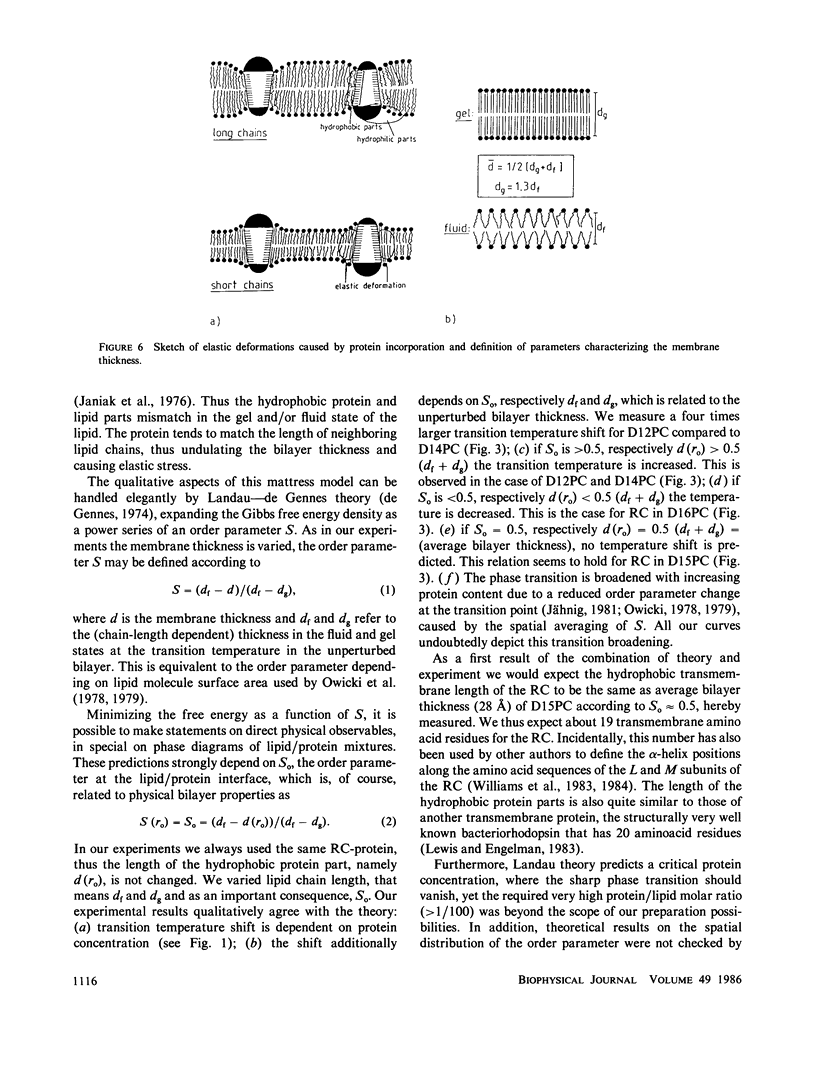
Reaction-center proteins of Rhodopseudomonas Sphaeroides reconstituted into phosphatidylcholine vesicles shift and broaden the fluid-gel transition of the lipid bilayer. The amount of broadening and temperature shift of the transition depend both on protein concentration and on lipid chain length. In particular, the direction of the transition temperature shift is very sensitive to lipid chain length. Electron micrographs show homogeneous protein distribution on the fluid surface whereas the solid phase contains protein aggregates the type depending on chain length. The results can qualitatively be understood in the framework of a mattress model of lipid/protein interactions in membranes.
Full text
PDF
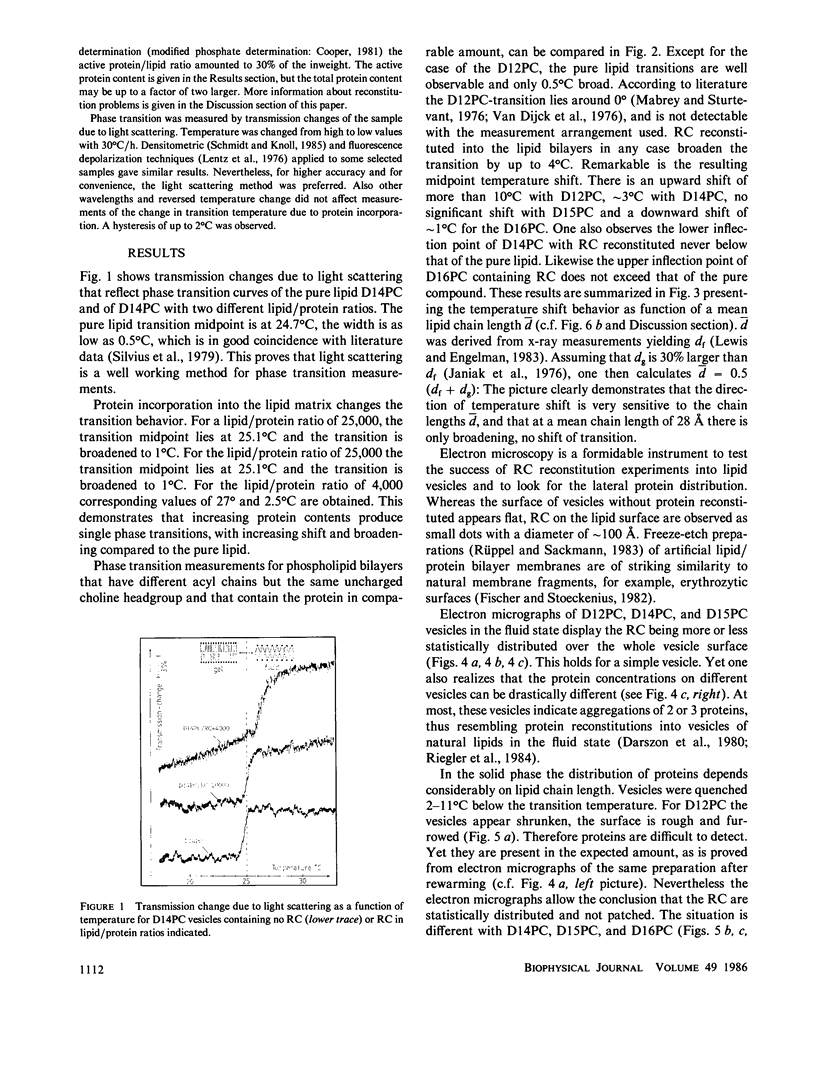
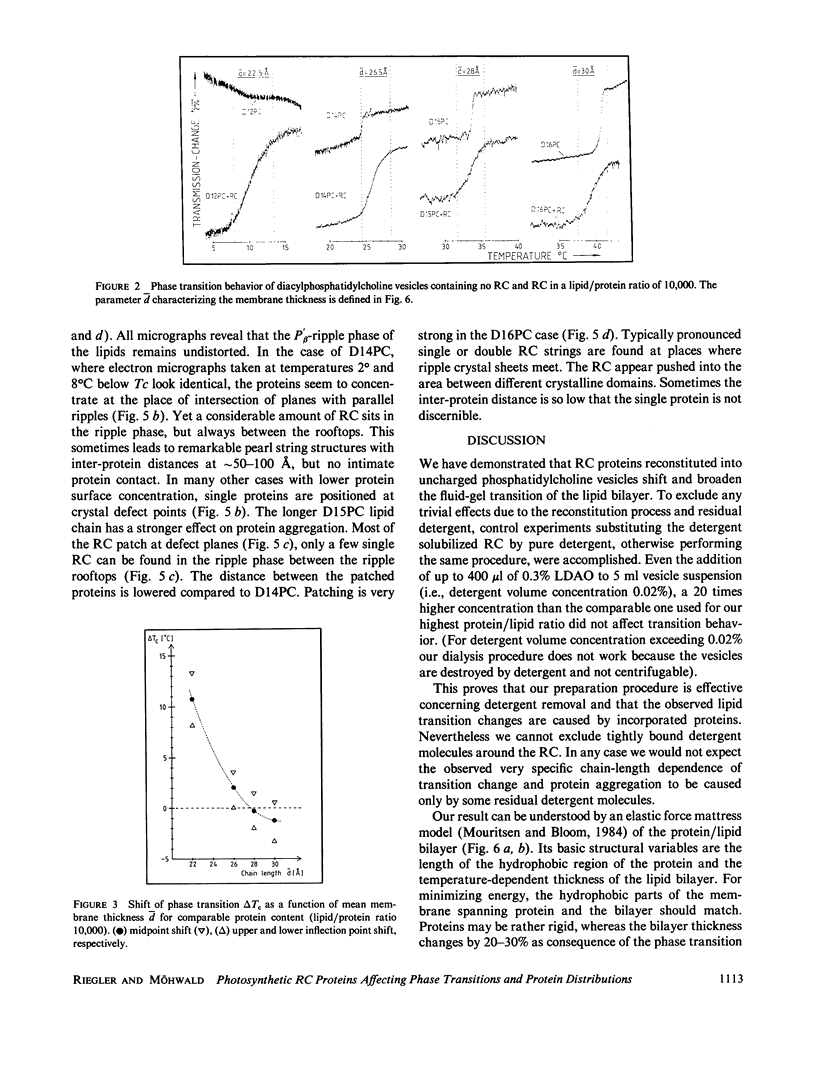
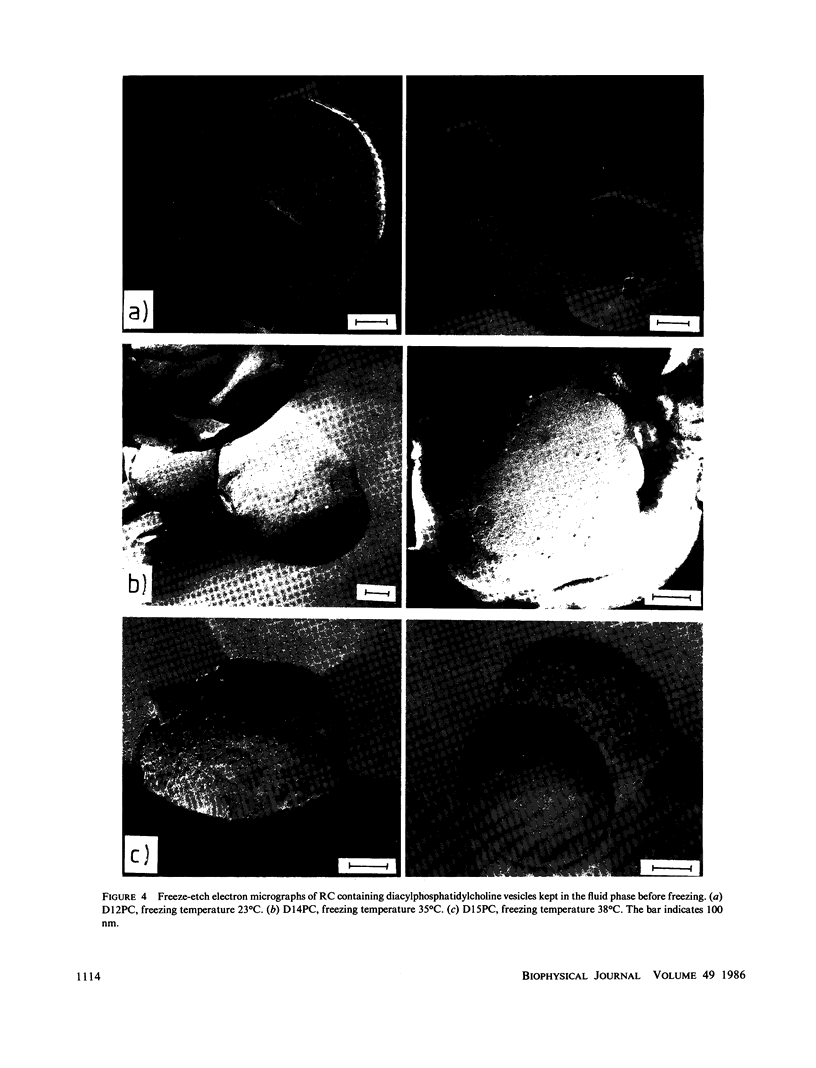


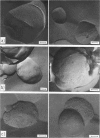
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Darszon A., Vandenberg C. A., Schönfeld M., Ellisman M. H., Spitzer N. C., Montal M. Reassembly of protein-lipid complexes into large bilayer vesicles: perspectives for membrane reconstitution. Proc Natl Acad Sci U S A. 1980 Jan;77(1):239–243. doi: 10.1073/pnas.77.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisenhofer J., Epp O., Miki K., Huber R., Michel H. X-ray structure analysis of a membrane protein complex. Electron density map at 3 A resolution and a model of the chromophores of the photosynthetic reaction center from Rhodopseudomonas viridis. J Mol Biol. 1984 Dec 5;180(2):385–398. doi: 10.1016/s0022-2836(84)80011-x. [DOI] [PubMed] [Google Scholar]
- Janiak M. J., Small D. M., Shipley G. G. Nature of the Thermal pretransition of synthetic phospholipids: dimyristolyl- and dipalmitoyllecithin. Biochemistry. 1976 Oct 19;15(21):4575–4580. doi: 10.1021/bi00666a005. [DOI] [PubMed] [Google Scholar]
- Jähnig F. Critical effects from lipid-protein interaction in membranes. I. Theoretical description. Biophys J. 1981 Nov;36(2):329–345. doi: 10.1016/S0006-3495(81)84735-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jähnig F. Critical effects from lipid-protein interaction in membranes. II. Interpretation of experimental results. Biophys J. 1981 Nov;36(2):347–357. doi: 10.1016/S0006-3495(81)84736-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentz B. R., Barenholz Y., Thompson T. E. Fluorescence depolarization studies of phase transitions and fluidity in phospholipid bilayers. 1. Single component phosphatidylcholine liposomes. Biochemistry. 1976 Oct 5;15(20):4521–4528. doi: 10.1021/bi00665a029. [DOI] [PubMed] [Google Scholar]
- Lewis B. A., Engelman D. M. Bacteriorhodopsin remains dispersed in fluid phospholipid bilayers over a wide range of bilayer thicknesses. J Mol Biol. 1983 May 15;166(2):203–210. doi: 10.1016/s0022-2836(83)80006-0. [DOI] [PubMed] [Google Scholar]
- Lewis B. A., Engelman D. M. Lipid bilayer thickness varies linearly with acyl chain length in fluid phosphatidylcholine vesicles. J Mol Biol. 1983 May 15;166(2):211–217. doi: 10.1016/s0022-2836(83)80007-2. [DOI] [PubMed] [Google Scholar]
- Mabrey S., Sturtevant J. M. Investigation of phase transitions of lipids and lipid mixtures by sensitivity differential scanning calorimetry. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3862–3866. doi: 10.1073/pnas.73.11.3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder M., Frisch H. L., Langer J. S., McConnell H. M. Theory of the intermediate rippled phase of phospholipid bilayers. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6559–6561. doi: 10.1073/pnas.81.20.6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouritsen O. G., Bloom M. Mattress model of lipid-protein interactions in membranes. Biophys J. 1984 Aug;46(2):141–153. doi: 10.1016/S0006-3495(84)84007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owicki J. C., McConnell H. M. Theory of protein-lipid and protein-protein interactions in bilayer membranes. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4750–4754. doi: 10.1073/pnas.76.10.4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owicki J. C., Springgate M. W., McConnell H. M. Theoretical study of protein--lipid interactions in bilayer membranes. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1616–1619. doi: 10.1073/pnas.75.4.1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riegler J., Peschke J., Möhwald H. Two-dimensional electron transfer from cytochrome C to photosynthetic reaction centers. Biochem Biophys Res Commun. 1984 Dec 14;125(2):592–599. doi: 10.1016/0006-291x(84)90580-1. [DOI] [PubMed] [Google Scholar]
- Sackmann E., Kotulla R., Heiszler F. J. On the role of lipid-bilayer elasticity for the lipid-protein interaction and the indirect protein-protein coupling. Can J Biochem Cell Biol. 1984 Aug;62(8):778–788. doi: 10.1139/o84-099. [DOI] [PubMed] [Google Scholar]
- Silvius J. R., Read B. D., McElhaney R. N. Thermotropic phase transitions of phosphatidylcholines with odd-numbered n-acyl chains. Biochim Biophys Acta. 1979 Jul 19;555(1):175–178. doi: 10.1016/0005-2736(79)90081-6. [DOI] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Szoka F., Jr, Papahadjopoulos D. Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4194–4198. doi: 10.1073/pnas.75.9.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijck P. W., De Kruijff B., Van Deenen L. L., De Gier J., Demel R. A. The preference of cholesterol for phosphatidylcholine in mixed phosphatidylcholine-phosphatidylethanolamine bilayers. Biochim Biophys Acta. 1976 Dec 2;455(2):576–587. doi: 10.1016/0005-2736(76)90326-6. [DOI] [PubMed] [Google Scholar]
- Williams J. C., Steiner L. A., Feher G., Simon M. I. Primary structure of the L subunit of the reaction center from Rhodopseudomonas sphaeroides. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7303–7307. doi: 10.1073/pnas.81.23.7303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. C., Steiner L. A., Ogden R. C., Simon M. I., Feher G. Primary structure of the M subunit of the reaction center from Rhodopseudomonas sphaeroides. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6505–6509. doi: 10.1073/pnas.80.21.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]