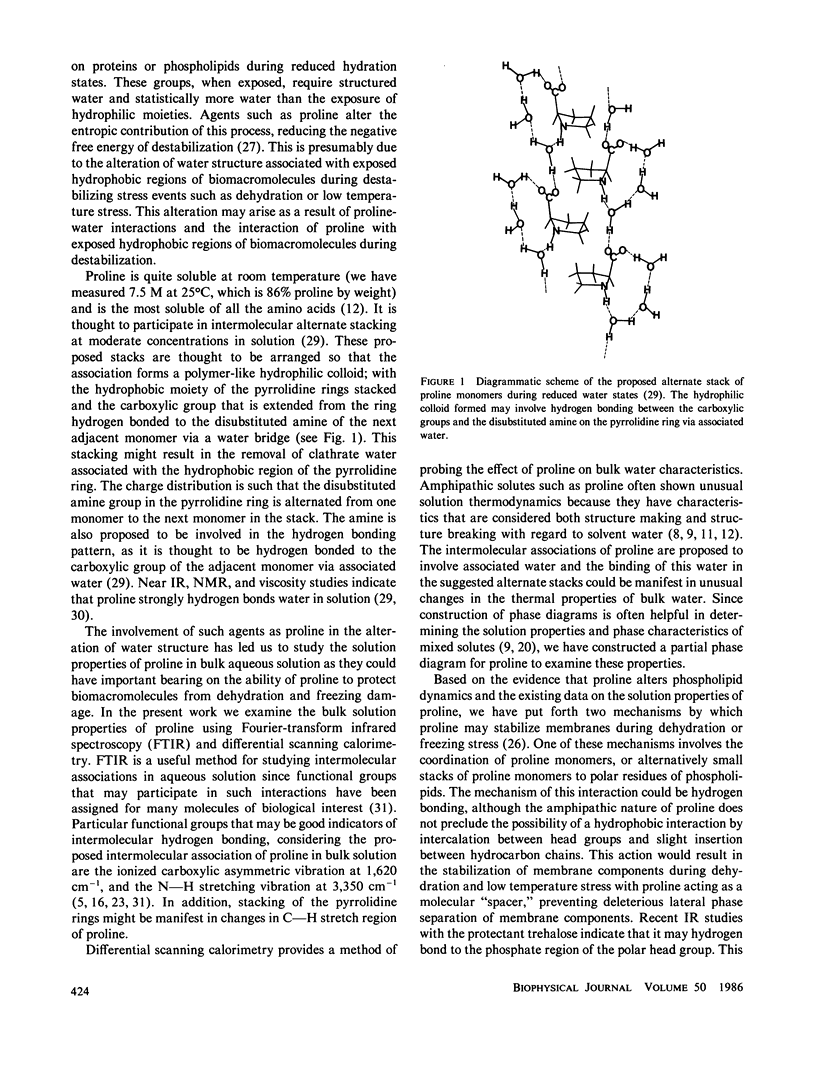
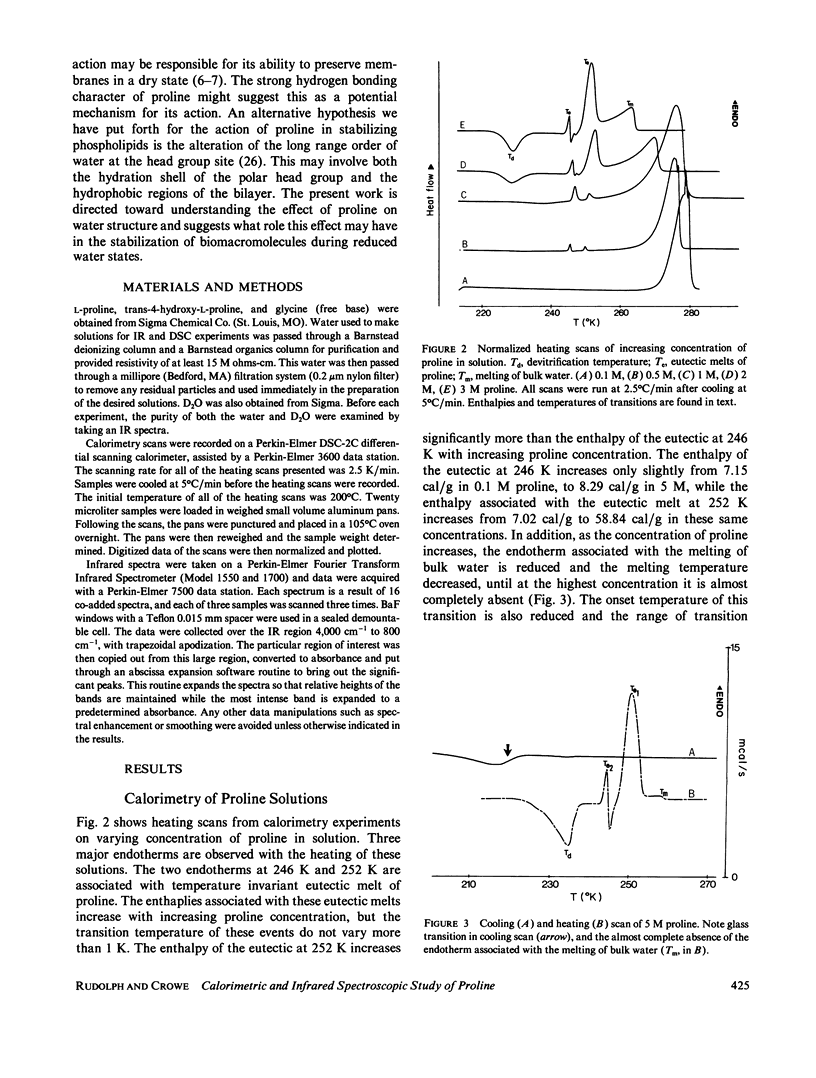
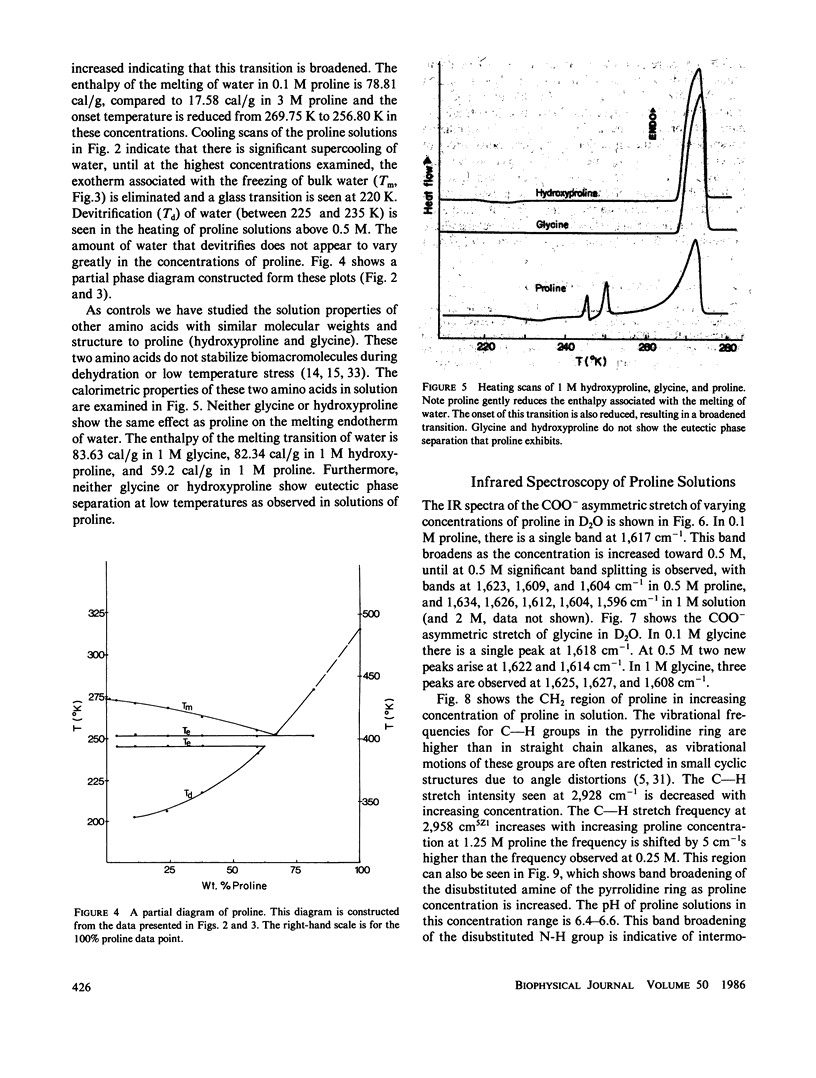
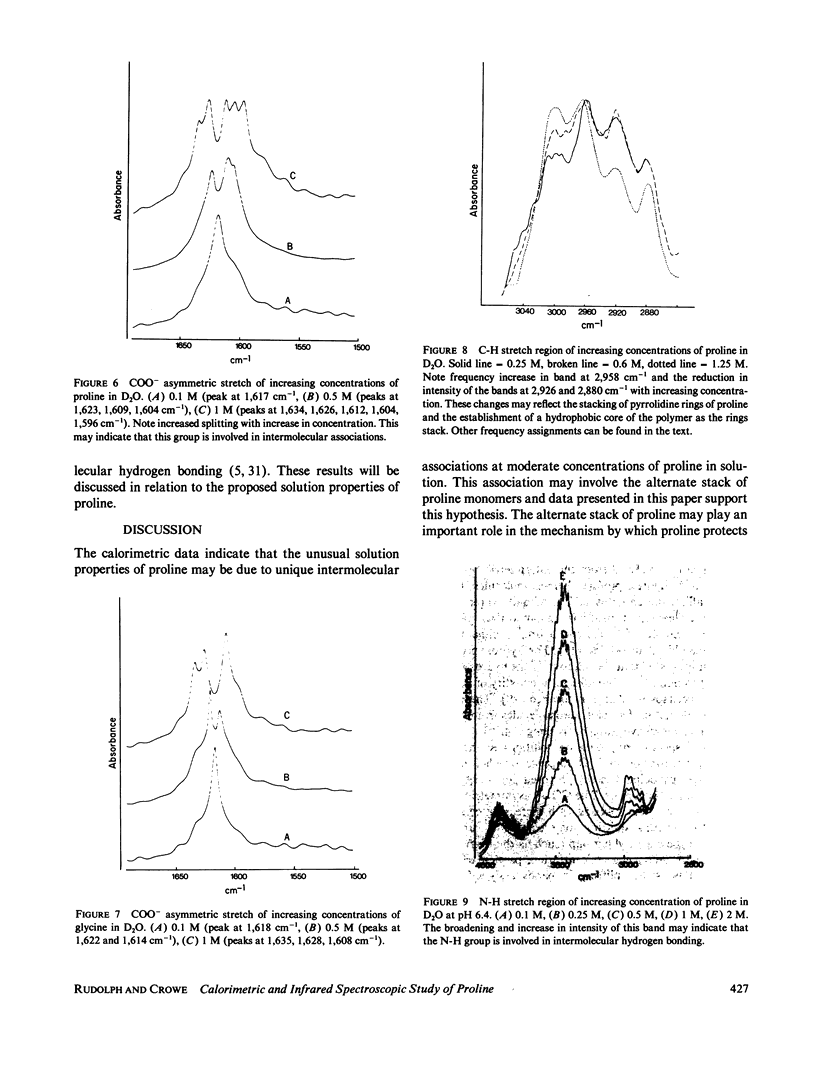
Abstract
We have studied the calorimetric and infrared spectroscopic properties of the amino acid proline which has been implicated in the stabilization of biomacromolecules during reduced water states. It has been suggested that the ability of this molecule to protect biomacromolecules during these stress states may be related to the formation of polymeric aggregates of proline monomers in solution. The structure of this aggregate is thought to be an alternates stack, forming a hydrophilic colloid-like polymer which is thought to interact with hydrophobic moieties of biomacromolecules, reducing the exposed hydrophobic area during reduced water conditions. Calorimetric data presented in this work show that in increasing concentration of proline in solution the enthalpy associated with the melting of bulk water is greatly reduced, indicating strong hydrogen bonding character of proline in aqueous solution. Proline shows two eutectic phase separations at moderate concentrations and one of these eutectics may be the proposed intermolecular state. A partial phase diagram for proline is presented. Fourier-transform infrared spectroscopic data indicate that the COO- asymmetric stretch of proline shows marked splitting with increasing proline concentration. This suggests that the carboxylate is in different environments, with the high energy vibrations representing COO- groups which are participating in the hydrogen bonding pattern associated with the formation of the intermolecular stack. Changes in the CH2 asymmetric and symmetric stretches of the pyrrolidine rings of proline are consistent with the proposed stack structure. We also suggest a possible mechanism by which these intermolecular associations may be important in the protection of biomacromolecules during reduced water states.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Casal H. L., Mantsch H. H. Polymorphic phase behaviour of phospholipid membranes studied by infrared spectroscopy. Biochim Biophys Acta. 1984 Dec 4;779(4):381–401. doi: 10.1016/0304-4157(84)90017-0. [DOI] [PubMed] [Google Scholar]
- Crowe J. H., Crowe L. M., Chapman D. Infrared spectroscopic studies on interactions of water and carbohydrates with a biological membrane. Arch Biochem Biophys. 1984 Jul;232(1):400–407. doi: 10.1016/0003-9861(84)90555-1. [DOI] [PubMed] [Google Scholar]
- Franks F. Solute--water interactions: do polyhydroxy compounds after the properties of water? Cryobiology. 1983 Jun;20(3):335–345. doi: 10.1016/0011-2240(83)90022-6. [DOI] [PubMed] [Google Scholar]
- Gaber B. P., Peticolas W. L. On the quantitative interpretation of biomembrane structure by Raman spectroscopy. Biochim Biophys Acta. 1977 Mar 1;465(2):260–274. doi: 10.1016/0005-2736(77)90078-5. [DOI] [PubMed] [Google Scholar]
- Heber U., Tyankova L., Santarius K. A. Stabilization and inactivation of biological membranes during freezing in the presence of amino acids. Biochim Biophys Acta. 1971 Aug 13;241(2):578–592. doi: 10.1016/0005-2736(71)90056-3. [DOI] [PubMed] [Google Scholar]
- Herlinger A. W., Long T. V., 2nd Laser-Raman and infrared spectra of amino acids and their metal complexes. 3. Proline and bisprolinato complexes. J Am Chem Soc. 1970 Nov 4;92(22):6481–6486. doi: 10.1021/ja00725a016. [DOI] [PubMed] [Google Scholar]
- Le Rudulier D., Bernard T., Goas G., Hamelin J. Osmoregulation in Klebsiella pneumoniae: enhancement of anaerobic growth and nitrogen fixation under stress by proline betaine, gamma-butyrobetaine, and other related compounds. Can J Microbiol. 1984 Mar;30(3):299–305. doi: 10.1139/m84-045. [DOI] [PubMed] [Google Scholar]
- Le Rudulier D., Strom A. R., Dandekar A. M., Smith L. T., Valentine R. C. Molecular biology of osmoregulation. Science. 1984 Jun 8;224(4653):1064–1068. doi: 10.1126/science.224.4653.1064. [DOI] [PubMed] [Google Scholar]
- Maggio B., Lucy J. A. Interactions of water-soluble fusogens with phospholipids in monolayers. FEBS Lett. 1978 Oct 15;94(2):301–304. doi: 10.1016/0014-5793(78)80962-4. [DOI] [PubMed] [Google Scholar]
- Pahlich E., Kerres R., Jäger H. J. Influence of Water Stress on the Vacuole/Extravacuole Distribution of Proline in Protoplasts of Nicotiana rustica. Plant Physiol. 1983 Jun;72(2):590–591. doi: 10.1104/pp.72.2.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph A. S., Crowe J. H., Crowe L. M. Effects of three stabilizing agents--proline, betaine, and trehalose--on membrane phospholipids. Arch Biochem Biophys. 1986 Feb 15;245(1):134–143. doi: 10.1016/0003-9861(86)90197-9. [DOI] [PubMed] [Google Scholar]
- Rudolph A. S., Crowe J. H. Membrane stabilization during freezing: the role of two natural cryoprotectants, trehalose and proline. Cryobiology. 1985 Aug;22(4):367–377. doi: 10.1016/0011-2240(85)90184-1. [DOI] [PubMed] [Google Scholar]
- Schobert B. Is there an osmotic regulatory mechanism in algae and higher plants? J Theor Biol. 1977 Sep 7;68(1):17–26. doi: 10.1016/0022-5193(77)90224-7. [DOI] [PubMed] [Google Scholar]
- Schobert B., Tschesche H. Unusual solution properties of proline and its interaction with proteins. Biochim Biophys Acta. 1978 Jun 15;541(2):270–277. doi: 10.1016/0304-4165(78)90400-2. [DOI] [PubMed] [Google Scholar]
- Tyankova L. The effect of amino acids on thylakoid membranes during freezing as influenced by side chain and position on the amino group. Biochim Biophys Acta. 1972 Jul 3;274(1):75–82. doi: 10.1016/0005-2736(72)90282-9. [DOI] [PubMed] [Google Scholar]
- Withers L. A., King P. J. Proline: A Novel Cryoprotectant for the Freeze Preservation of Cultured Cells of Zea mays L. Plant Physiol. 1979 Nov;64(5):675–678. doi: 10.1104/pp.64.5.675. [DOI] [PMC free article] [PubMed] [Google Scholar]


