Abstract
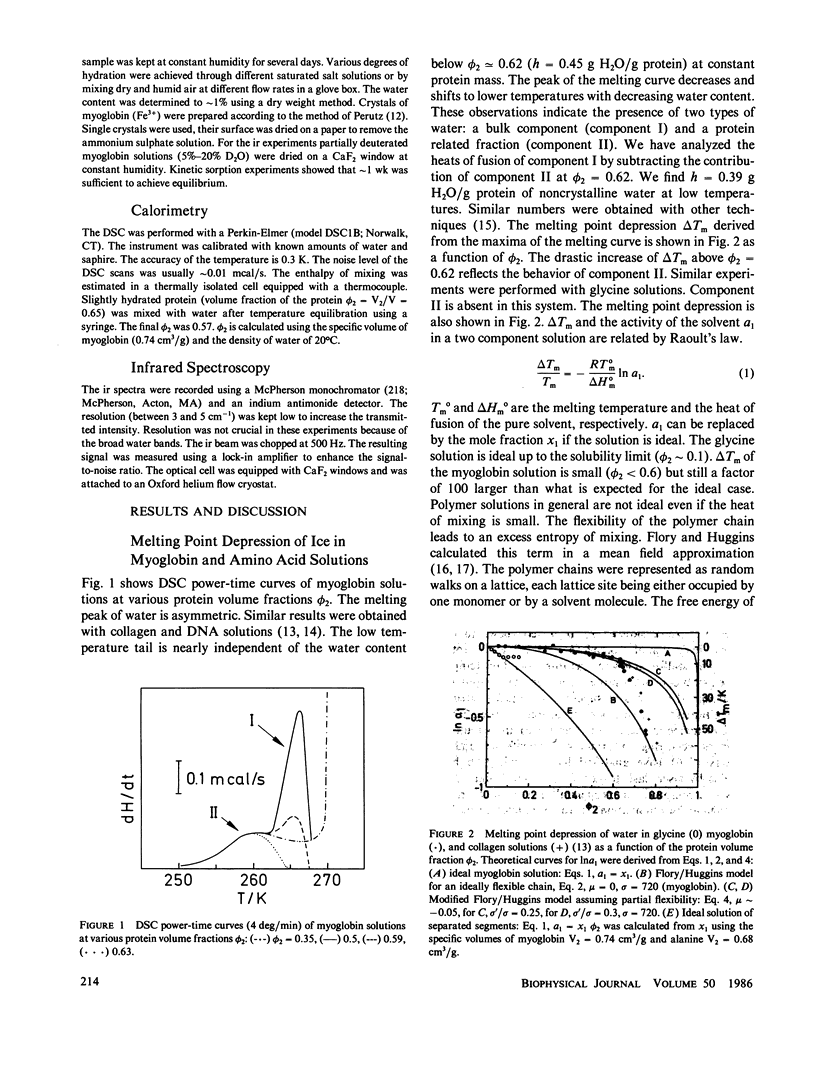
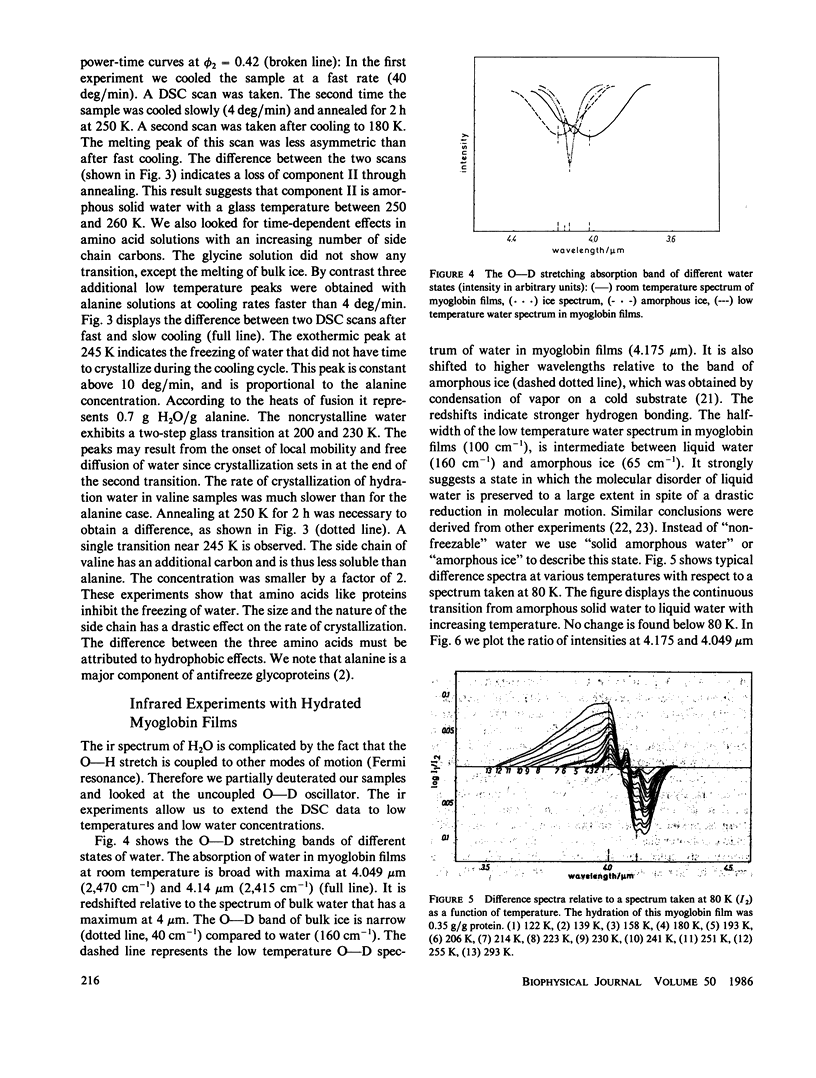
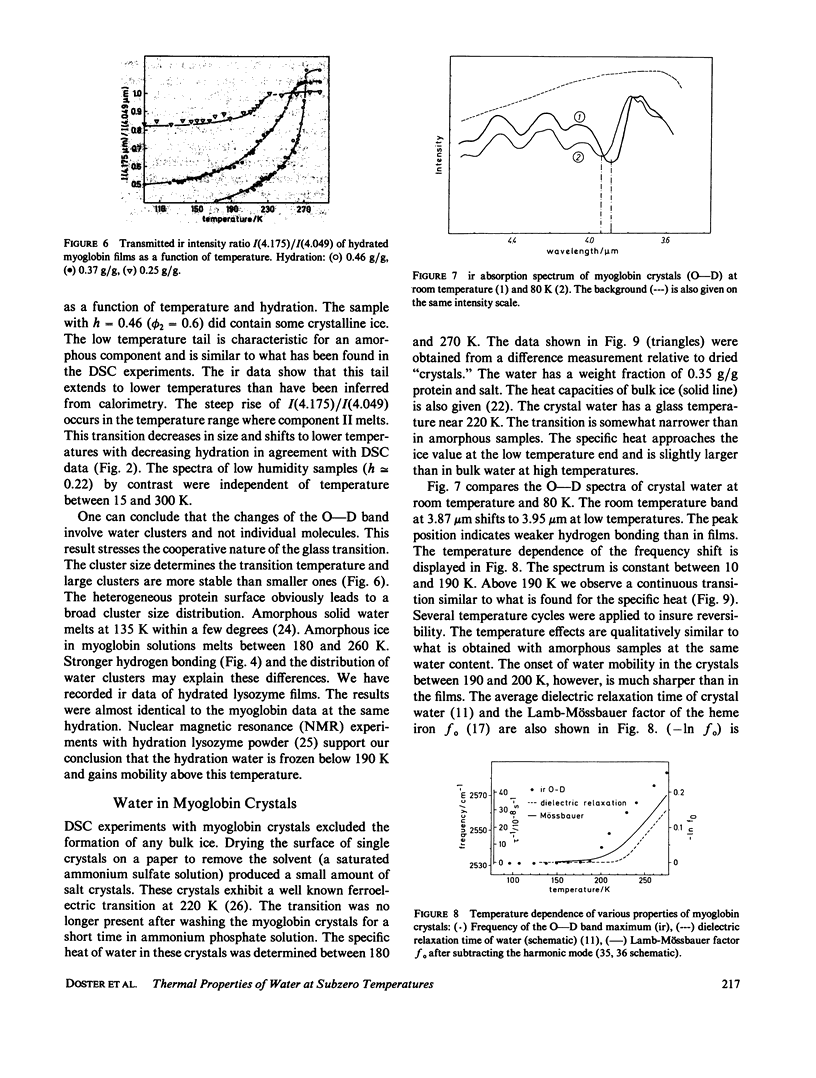
The water of hydration in myoglobin crystals and solutions was studied at subzero temperatures by calorimetry and infrared spectroscopy (ir). For comparison we also investigated glycine, DL-alanine and DL-valine solutions. The hydration water remains amorphous at low temperatures. We find a broad glass transition between 180 and 270 K depending on the degree of hydration. The ice component shows a noncolligative melting point depression that is attributed to a finite conformational flexibility. The ir spectrum and the specific heat of water in myoglobin crystals was determined for the first time between 180 and 290 K. The glass transition in crystals is qualitatively similar to what is found in amorphous samples at the same water content. These data are compared with Mössbauer experiments and dielectric relaxation of water in myoglobin crystals. The similar temperature dependencies suggest a cross correlation between structural fluctuations and the thermal motion of crystal water. A hydrogen bond network model is proposed to explain these features. The essential ingredients are cooperativity and a distribution of hydrogen-bonded clusters.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austin R. H., Beeson K. W., Eisenstein L., Frauenfelder H., Gunsalus I. C. Dynamics of ligand binding to myoglobin. Biochemistry. 1975 Dec 2;14(24):5355–5373. doi: 10.1021/bi00695a021. [DOI] [PubMed] [Google Scholar]
- Brown R. A., Yeh Y., Burcham T. S., Feeney R. E. Direct evidence for antifreeze glycoprotein adsorption onto an ice surface. Biopolymers. 1985 Jul;24(7):1265–1270. doi: 10.1002/bip.360240713. [DOI] [PubMed] [Google Scholar]
- Careri G., Fasella P., Gratton E. Enzyme dynamics: the statistical physics approach. Annu Rev Biophys Bioeng. 1979;8:69–97. doi: 10.1146/annurev.bb.08.060179.000441. [DOI] [PubMed] [Google Scholar]
- Chothia C. The nature of the accessible and buried surfaces in proteins. J Mol Biol. 1976 Jul 25;105(1):1–12. doi: 10.1016/0022-2836(76)90191-1. [DOI] [PubMed] [Google Scholar]
- Doster W., Beece D., Bowne S. F., DiIorio E. E., Eisenstein L., Frauenfelder H., Reinisch L., Shyamsunder E., Winterhalter K. H., Yue K. T. Control and pH dependence of ligand binding to heme proteins. Biochemistry. 1982 Sep 28;21(20):4831–4839. doi: 10.1021/bi00263a001. [DOI] [PubMed] [Google Scholar]
- Frauenfelder H., Petsko G. A., Tsernoglou D. Temperature-dependent X-ray diffraction as a probe of protein structural dynamics. Nature. 1979 Aug 16;280(5723):558–563. doi: 10.1038/280558a0. [DOI] [PubMed] [Google Scholar]
- Haly A. R., Snaith J. W. Calorimetry of rat tail tendon collagen before and after denaturation: the heat of fusion of its absorbed water. Biopolymers. 1971;10(9):1681–1699. doi: 10.1002/bip.360100921. [DOI] [PubMed] [Google Scholar]
- Hartmann H., Parak F., Steigemann W., Petsko G. A., Ponzi D. R., Frauenfelder H. Conformational substates in a protein: structure and dynamics of metmyoglobin at 80 K. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4967–4971. doi: 10.1073/pnas.79.16.4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton B. D., Hsi E., Bryant R. G. 1H nuclear magnetic resonance relaxation of water on lysozyme powders. J Am Chem Soc. 1977 Dec 21;99(26):8483–8490. doi: 10.1021/ja00468a017. [DOI] [PubMed] [Google Scholar]
- Krupyanskii YuF, Parak F., Goldanskii V. I., Mössbauer R. L., Gaubman E. E., Engelmann H., Suzdalev I. P. Investigation of large intramolecular movement within metmyoglobin by Rayleigh scattering of Mössbauer radiation (RSMR). Z Naturforsch C. 1982 Jan-Feb;37(1-2):57–62. doi: 10.1515/znc-1982-1-211. [DOI] [PubMed] [Google Scholar]
- Kuntz I. D., Jr, Kauzmann W. Hydration of proteins and polypeptides. Adv Protein Chem. 1974;28:239–345. doi: 10.1016/s0065-3233(08)60232-6. [DOI] [PubMed] [Google Scholar]
- Mayo K. H., Kucheida D., Parak F., Chien J. C. Structural dynamics of human deoxyhemoglobin and hemochrome investigated by nuclear gamma resonance absorption (Mössbauer) spectroscopy. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5294–5296. doi: 10.1073/pnas.80.17.5294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler W., Schulten K. Theory of Mössbauer spectra of proteins fluctuating between conformational substates. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5719–5723. doi: 10.1073/pnas.81.18.5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parak F., Frolov E. N., Mössbauer R. L., Goldanskii V. I. Dynamics of metmyoglobin crystals investigated by nuclear gamma resonance absorption. J Mol Biol. 1981 Feb 5;145(4):825–833. doi: 10.1016/0022-2836(81)90317-x. [DOI] [PubMed] [Google Scholar]
- Richards F. M. Areas, volumes, packing and protein structure. Annu Rev Biophys Bioeng. 1977;6:151–176. doi: 10.1146/annurev.bb.06.060177.001055. [DOI] [PubMed] [Google Scholar]


