Abstract
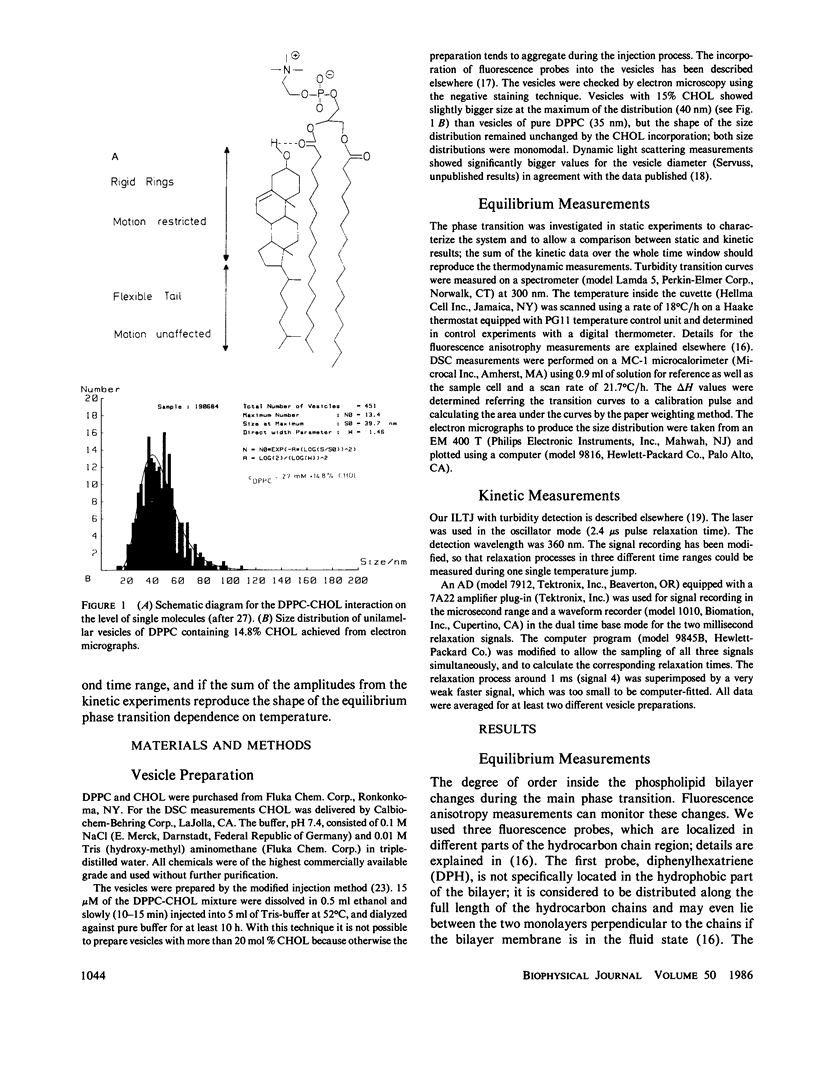
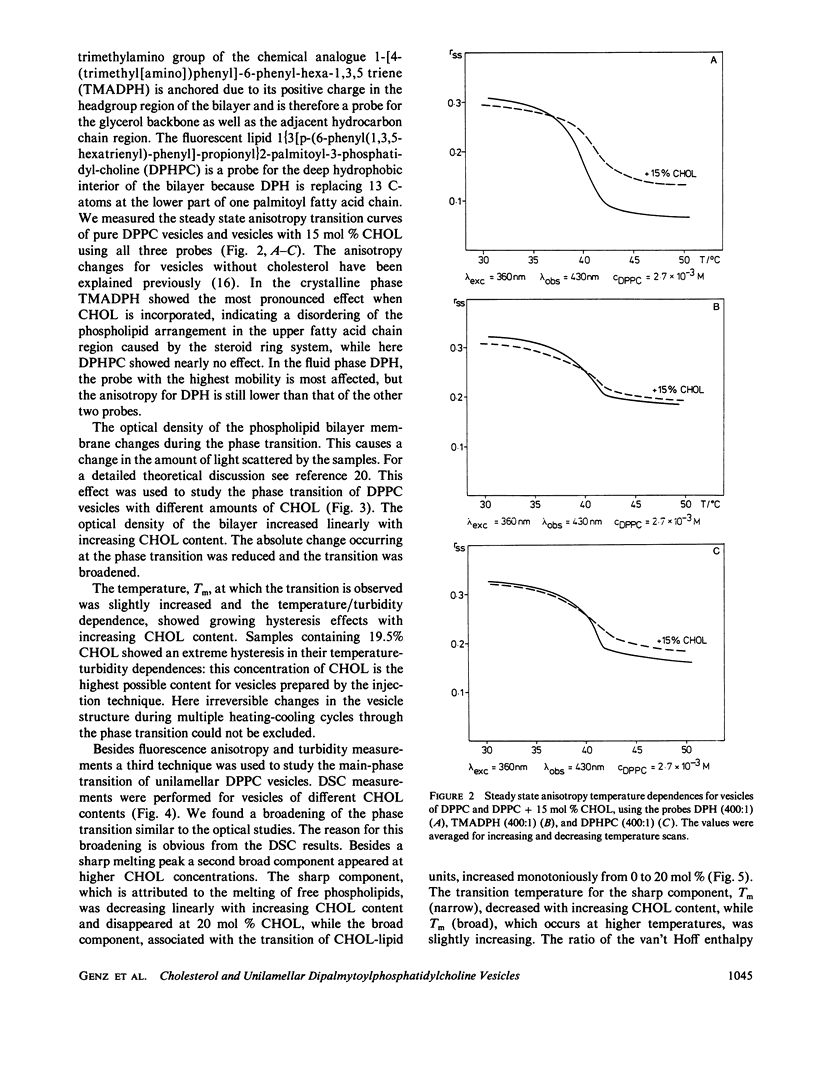
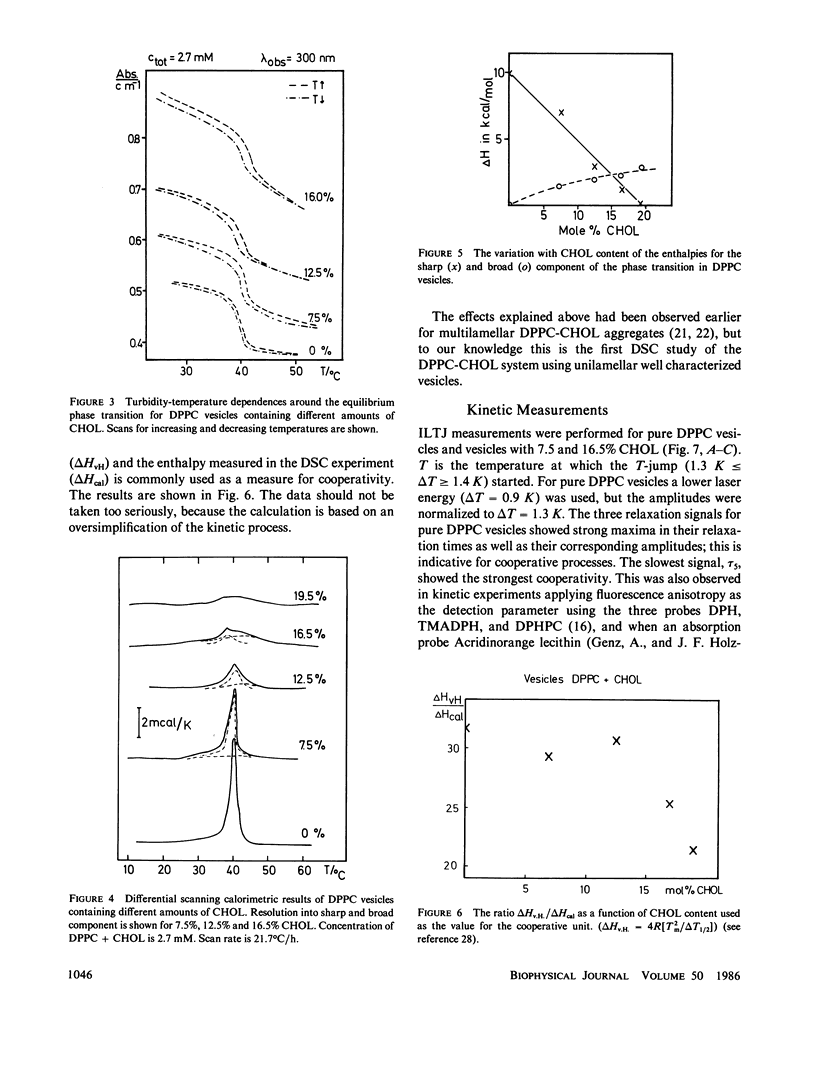
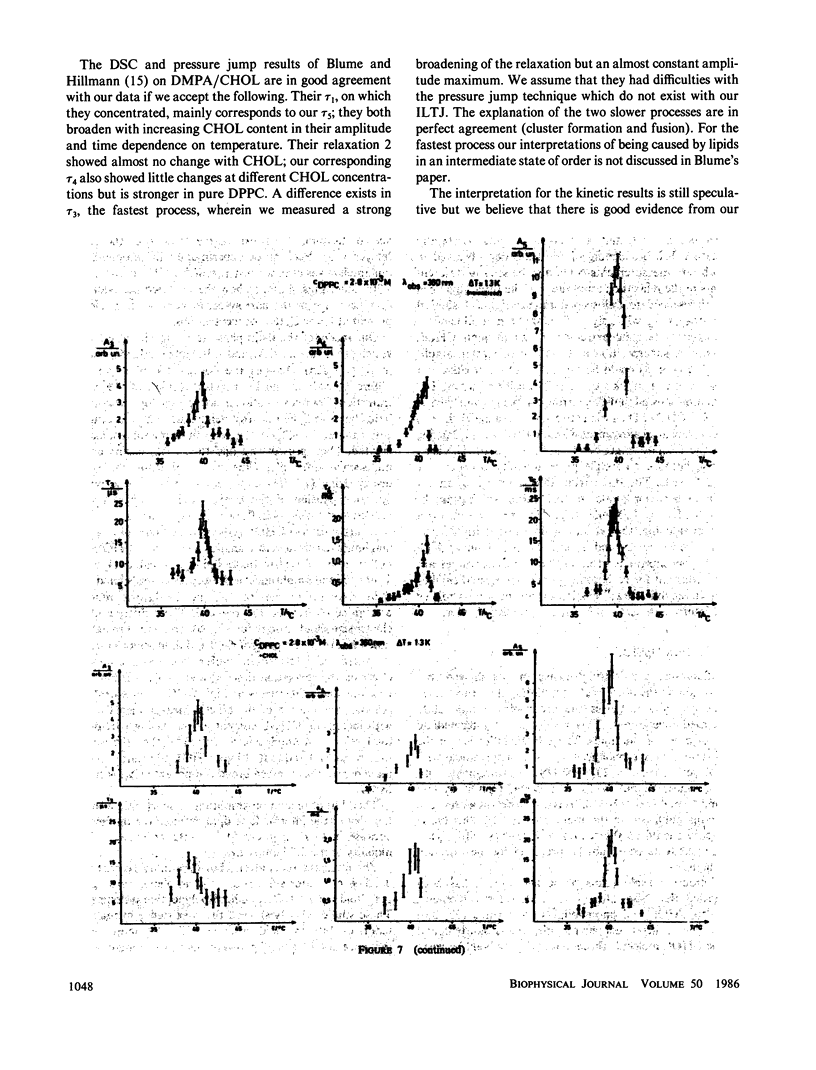
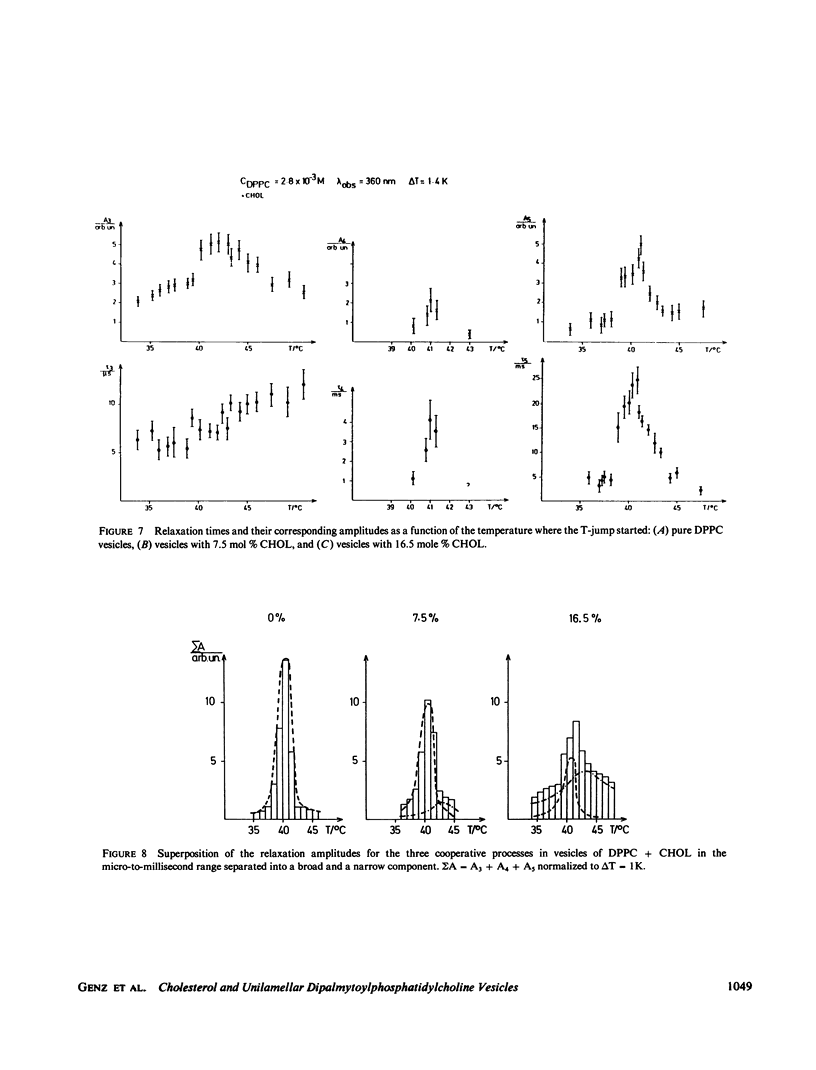
The influence of cholesterol (CHOL) on the main phase transition in single shell dipalmytoylphosphatidylcholine (DPPC) vesicles was investigated in equilibrium and kinetic experiments. CHOL increases the optical density and causes a slight hysteresis in turbidity transition curves. Static fluorescence anisotropy measurements showed interesting differences for three probes sensing different parts in the hydrophobic region of the phospholipid bilayer. Differential scanning calorimetry (DSC) peaks can be separated into a narrow and a broad component. The narrow component, which decreases linearly with increasing CHOL content and disappears at 20 mol %, is attributed to the transition of free phospholipid, while the broad component, being associated with the transition of CHOL-lipid units, increases monotoniously from 0 to 20%. Kinetic experiments were performed on our iodine-laser T-jump arrangement with turbidity detection. Three cooperative relaxation signals in the microsecond and millisecond time range were detected for pure DPPC vesicles as well as vesicles containing 7.5 and 16.5 mol % CHOL. All three relaxation processes were changed by CHOL: the superposition of the three relaxation amplitudes can be separated into a narrow and a broad component, as in DSC experiments. A speculative model is presented which assumes an inhomogeneous CHOL distribution fluctuating on a millisecond time scale in the temperature region of the main phase transition.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blume A., Griffin R. G. Carbon-13 and deuterium nuclear magnetic resonance study of the interaction of cholesterol with phosphatidylethanolamine. Biochemistry. 1982 Nov 23;21(24):6230–6242. doi: 10.1021/bi00267a031. [DOI] [PubMed] [Google Scholar]
- Blume A. Thermotropic behavior of phosphatidylethanolamine-cholesterol and phosphatidylethanolamine-phosphatidylcholine-cholesterol mixtures. Biochemistry. 1980 Oct 14;19(21):4908–4913. doi: 10.1021/bi00562a032. [DOI] [PubMed] [Google Scholar]
- Chong C. S., Colbow K. Light scattering and turbidity measurements on lipid vesicles. Biochim Biophys Acta. 1976 Jun 17;436(2):260–282. doi: 10.1016/0005-2736(76)90192-9. [DOI] [PubMed] [Google Scholar]
- Demel R. A., De Kruyff B. The function of sterols in membranes. Biochim Biophys Acta. 1976 Oct 26;457(2):109–132. doi: 10.1016/0304-4157(76)90008-3. [DOI] [PubMed] [Google Scholar]
- Estep T. N., Mountcastle D. B., Biltonen R. L., Thompson T. E. Studies on the anomalous thermotropic behavior of aqueous dispersions of dipalmitoylphosphatidylcholine-cholesterol mixtures. Biochemistry. 1978 May 16;17(10):1984–1989. doi: 10.1021/bi00603a029. [DOI] [PubMed] [Google Scholar]
- Hinz H. J., Sturtevant J. M. Calorimetric studies of dilute aqueous suspensions of bilayers formed from synthetic L- -lecithins. J Biol Chem. 1972 Oct 10;247(19):6071–6075. [PubMed] [Google Scholar]
- Jacobs R., Oldfield E. Deuterium nuclear magnetic resonance investigation of dimyristoyllecithin--dipalmitoyllecithin and dimyristoyllecithin--cholesterol mixtures. Biochemistry. 1979 Jul 24;18(15):3280–3285. doi: 10.1021/bi00582a013. [DOI] [PubMed] [Google Scholar]
- Kleemann W., McConnell H. M. Interactions of proteins and cholesterol with lipids in bilayer membranes. Biochim Biophys Acta. 1976 Jan 21;419(2):206–222. doi: 10.1016/0005-2736(76)90347-3. [DOI] [PubMed] [Google Scholar]
- Kremer J. M., Esker M. W., Pathmamanoharan C., Wiersema P. H. Vesicles of variable diameter prepared by a modified injection method. Biochemistry. 1977 Aug 23;16(17):3932–3935. doi: 10.1021/bi00636a033. [DOI] [PubMed] [Google Scholar]
- Lentz B. R., Barrow D. A., Hoechli M. Cholesterol-phosphatidylcholine interactions in multilamellar vesicles. Biochemistry. 1980 Apr 29;19(9):1943–1954. doi: 10.1021/bi00550a034. [DOI] [PubMed] [Google Scholar]
- Mabrey S., Mateo P. L., Sturtevant J. M. High-sensitivity scanning calorimetric study of mixtures of cholesterol with dimyristoyl- and dipalmitoylphosphatidylcholines. Biochemistry. 1978 Jun 13;17(12):2464–2468. doi: 10.1021/bi00605a034. [DOI] [PubMed] [Google Scholar]
- Presti F. T., Chan S. I. Cholesterol-phospholipid interaction in membranes. 1. Cholestane spin-label studies of phase behavior of cholesterol-phospholipid liposomes. Biochemistry. 1982 Aug 3;21(16):3821–3830. doi: 10.1021/bi00259a016. [DOI] [PubMed] [Google Scholar]
- Presti F. T., Pace R. J., Chan S. I. Cholesterol-phospholipid interaction in membranes. 2. Stoichiometry and molecular packing of cholesterol-rich domains. Biochemistry. 1982 Aug 3;21(16):3831–3835. doi: 10.1021/bi00259a017. [DOI] [PubMed] [Google Scholar]
- Recktenwald D. J., McConnell H. M. Phase equilibria in binary mixtures of phosphatidylcholine and cholesterol. Biochemistry. 1981 Jul 21;20(15):4505–4510. doi: 10.1021/bi00518a042. [DOI] [PubMed] [Google Scholar]
- Rogers J., Lee A. G., Wilton D. C. The organisation of cholesterol and ergosterol in lipid bilayers based on studies using non-perturbing fluorescent sterol probes. Biochim Biophys Acta. 1979 Mar 23;552(1):23–37. doi: 10.1016/0005-2736(79)90243-8. [DOI] [PubMed] [Google Scholar]
- Rubenstein J. L., Smith B. A., McConnell H. M. Lateral diffusion in binary mixtures of cholesterol and phosphatidylcholines. Proc Natl Acad Sci U S A. 1979 Jan;76(1):15–18. doi: 10.1073/pnas.76.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smutzer G., Yeagle P. L. A fluorescence anisotropy study on the phase behavior of dimyristoylphosphatidylcholine/cholesterol mixtures. Biochim Biophys Acta. 1985 Apr 11;814(2):274–280. doi: 10.1016/0005-2736(85)90445-6. [DOI] [PubMed] [Google Scholar]
- Snyder B., Freire E. Compositional domain structure in phosphatidylcholine--cholesterol and sphingomyelin--cholesterol bilayers. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4055–4059. doi: 10.1073/pnas.77.7.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tson- T. Y. Transport of 8-anilino-1-naphthalenesulfonate as a probe of the effect of cholesterol on the phospholipid bilayer structures. Biochemistry. 1975 Dec 16;14(25):5415–5417. doi: 10.1021/bi00696a005. [DOI] [PubMed] [Google Scholar]
- Vincent M., de Foresta B., Gallay J., Alfsen A. Fluorescence anisotropy decays of n-(9-anthroyloxy) fatty acids in dipalmitoyl phosphatidylcholine vesicles. Localization of the effects of cholesterol addition. Biochem Biophys Res Commun. 1982 Aug;107(3):914–921. doi: 10.1016/0006-291x(82)90610-6. [DOI] [PubMed] [Google Scholar]


