Abstract
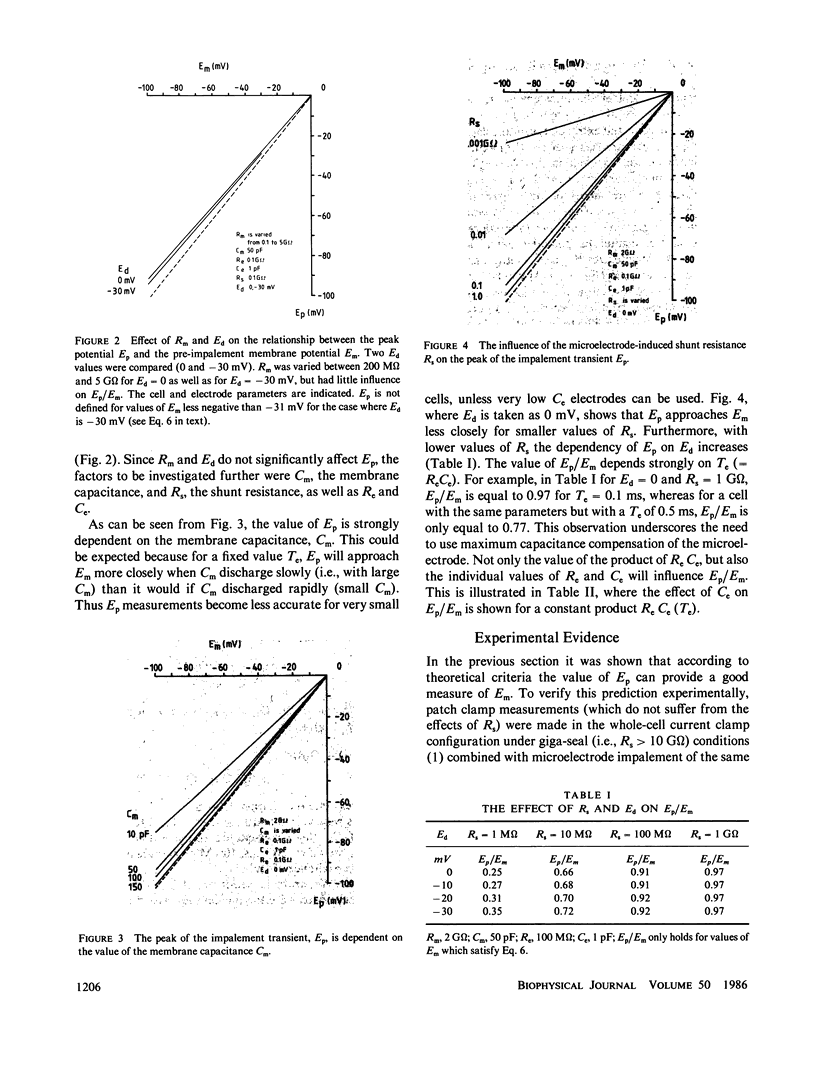
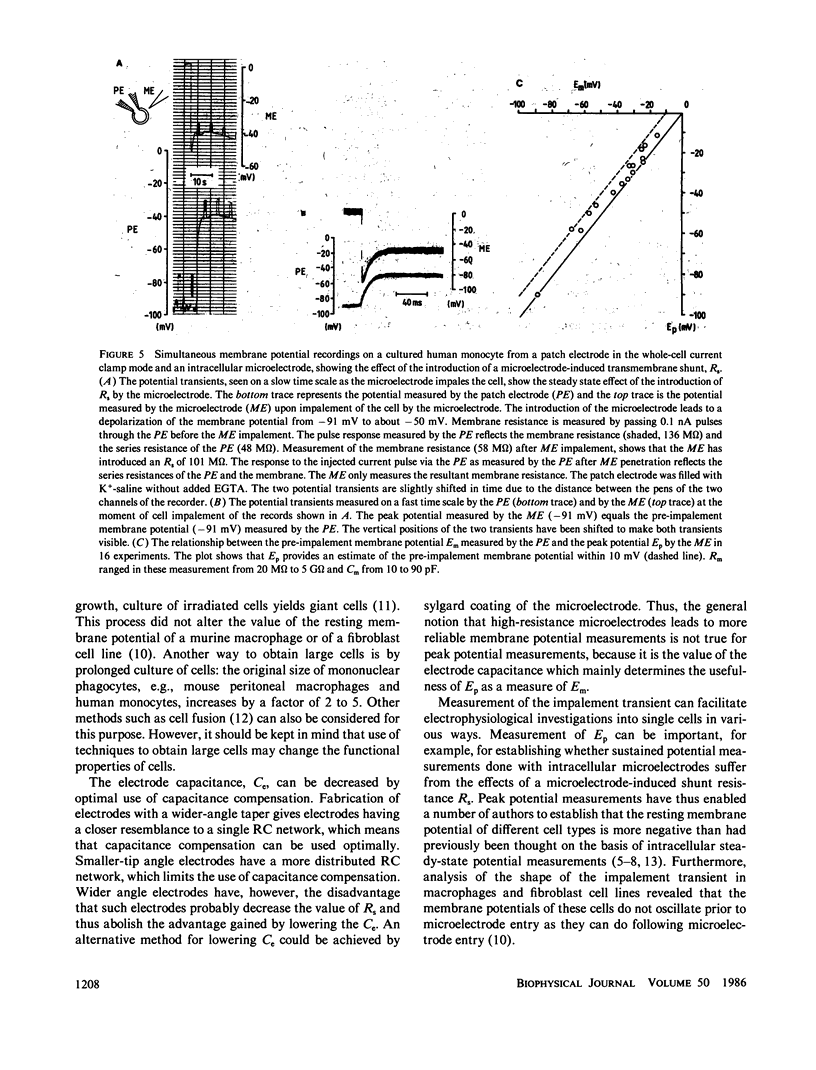
Microelectrode penetration of small cells leads to a sustained depolarization of the resting membrane potential due to a transmembrane shunt resistance (Rs) introduced by the microelectrode. This has led to underestimation of the resting membrane potential of various cell types. However, measurement of the fast potential transient occurring within the first few milliseconds after microelectrode penetration can provide information about pre-impalement membrane electrophysiological properties. We have analyzed an equivalent circuit of a microelectrode measurement to establish the conditions under which the peak of the impalement transients (Ep) approaches the pre-impalement resting membrane potential (Em) of small cells most closely. The simulation studies showed that this is the case when the capacitance of the microelectrode is low and the membrane capacitance of the cell high. In experiments performed to assess the reliability of Ep as a measure of Em, whole-cell patch clamp measurements were performed in the current clamp mode to monitor, free from the effects of Rs, Em in cultured human monocytes. Microelectrode impalement of such patch clamped cells and measurement of Ep made it possible to detect correlation between Ep and Em and showed that for small cells such as human monocytes Ep is on average 6 mV less negative than the resting membrane potential.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blatt M. R., Slayman C. L. KCl leakage from microelectrodes and its impact on the membrane parameters of a nonexcitable cell. J Membr Biol. 1983;72(3):223–234. doi: 10.1007/BF01870589. [DOI] [PubMed] [Google Scholar]
- Chambers E. L., de Armendi J. Membrane potential, action potential and activation potential of eggs of the sea urchin, Lytechinus variegatus. Exp Cell Res. 1979 Aug;122(1):203–218. doi: 10.1016/0014-4827(79)90575-5. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Ince C., Leijh P. C., Meijer J., Van Bavel E., Ypey D. L. Oscillatory hyperpolarizations and resting membrane potentials of mouse fibroblast and macrophage cell lines. J Physiol. 1984 Jul;352:625–635. doi: 10.1113/jphysiol.1984.sp015313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ince C., Ypey D. L., Van Furth R., Verveen A. A. Estimation of the membrane potential of cultured macrophages from the fast potential transient upon microelectrode entry. J Cell Biol. 1983 Mar;96(3):796–801. doi: 10.1083/jcb.96.3.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ince C., van Dissel J. T., Diesselhoff M. M. A teflon culture dish for high-magnification microscopy and measurements in single cells. Pflugers Arch. 1985 Mar;403(3):240–244. doi: 10.1007/BF00583594. [DOI] [PubMed] [Google Scholar]
- Neher E., Sakmann B. Single-channel currents recorded from membrane of denervated frog muscle fibres. Nature. 1976 Apr 29;260(5554):799–802. doi: 10.1038/260799a0. [DOI] [PubMed] [Google Scholar]
- Persechini P. M., Araujo E. G., Oliveira-Castro G. M. Electrophysiology of phagocytic membranes: induction of slow membrane hyperpolarizations in macrophages and macrophage polykaryons by intracellular calcium injection. J Membr Biol. 1981;61(2):81–90. doi: 10.1007/BF02007634. [DOI] [PubMed] [Google Scholar]
- WHITMORE G. F., TILL J. E., GWATKIN R. B., SIMINOVITCH L., GRAHAM A. F. Increase of cellular constituents in x-irradiated mammalian cells. Biochim Biophys Acta. 1958 Dec;30(3):583–590. doi: 10.1016/0006-3002(58)90105-7. [DOI] [PubMed] [Google Scholar]


