Abstract
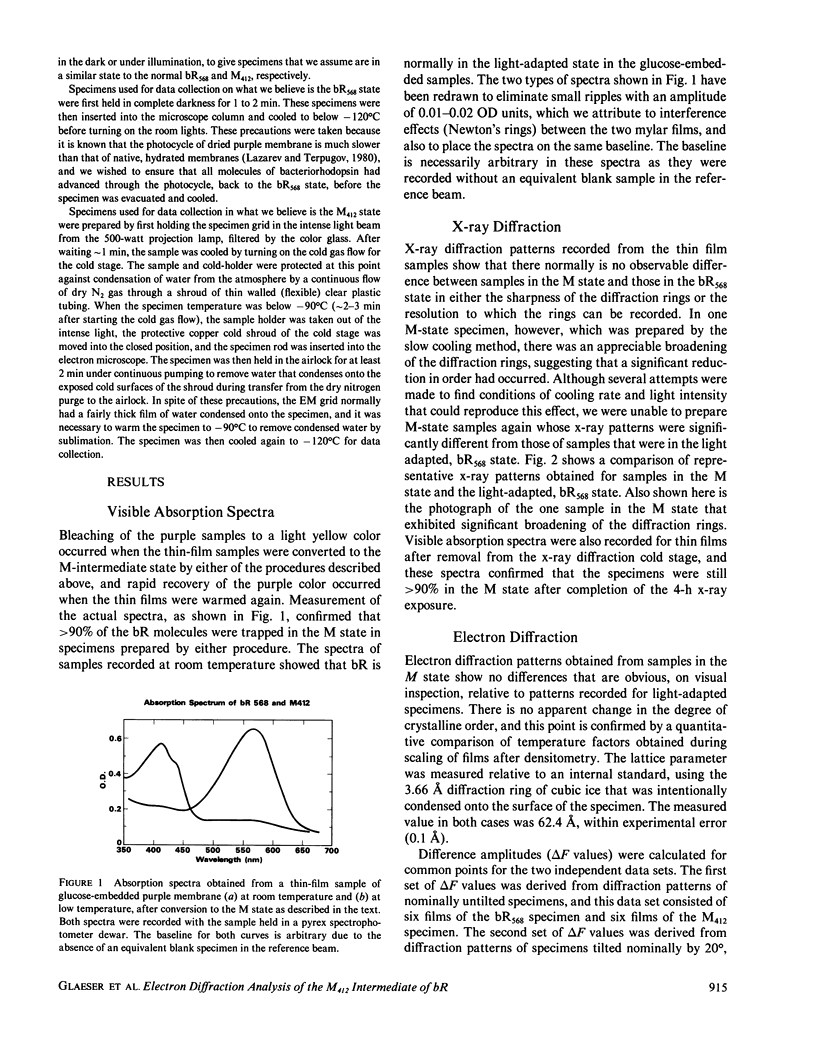
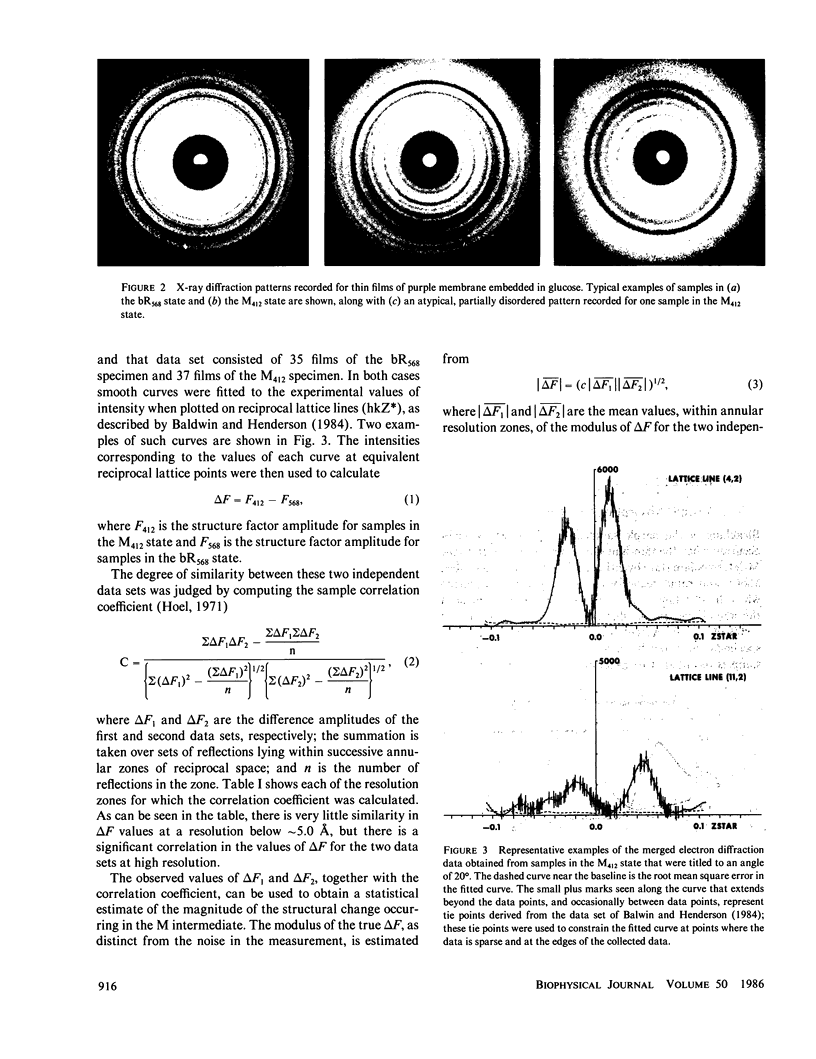
High resolution electron diffraction data have been recorded for glucose-embedded purple membrane specimens in which bacteriorhodopsin (bR) has been trapped by cooling slowly to below--100 degrees C under continuous illumination. Thin films (OD approximately 0.7) of glucose-embedded membranes, prepared as a control, showed virtually 100% conversion to the M state, and stacks of such thin film specimens gave very similar x-ray diffraction patterns in the bR568 and the M412 state in most experiments. To be certain that any measured differences in diffraction intensity would be real, two independent sets of electron diffraction intensities were recorded for near-equatorial, i.e. (hkO), reflections. Little correlation was indeed observed between these two sets for delta F values at low resolution (15-5.0 A, 49 reflections), but the correlation coefficient is approximately 0.3 at high resolution (5.0-3.3 A, 218 reflections). Thus, while most of the measured difference is error, the mean delta F and the correlation coefficient can be used to estimate the smaller, true delta F due to structural changes occurring in the M state. The magnitude of this estimated true mean delta F is equal to what would be produced if approximately five to seven nonhydrogen atoms were moved to structurally uncorrelated (i.e., new) positions in the M state. Movements of a few amino acid side chains, and repositioning of atoms of the retinal group and the associated lysine side chain after trans-cis isomerization, are the most probable causes of the observed intensity changes in the M state. The difference Fourier map, calculated in projection at 3.5-A resolution, shows only very small peaks, the largest of which are confined, however, to the region of the protein.
Full text
PDF

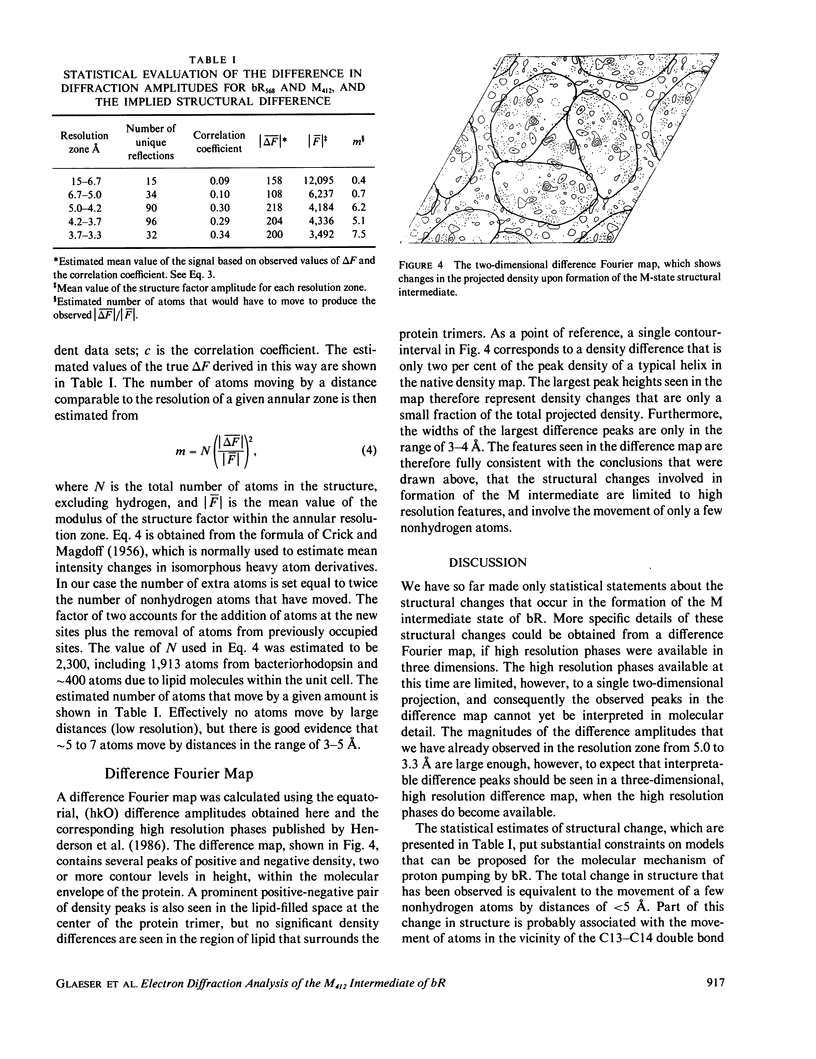



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahl P. L., Cone R. A. Light activates rotations of bacteriorhodopsin in the purple membrane. Biophys J. 1984 Jun;45(6):1039–1049. doi: 10.1016/S0006-3495(84)84251-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becher B., Tokunaga F., Ebrey T. G. Ultraviolet and visible absorption spectra of the purple membrane protein and the photocycle intermediates. Biochemistry. 1978 Jun 13;17(12):2293–2300. doi: 10.1021/bi00605a006. [DOI] [PubMed] [Google Scholar]
- Bogomolni R. A., Stubbs L., Lanyi J. K. Illumination-dependent changes in the intrinsic fluorescence of bacteriorhodopsin. Biochemistry. 1978 Mar 21;17(6):1037–1041. doi: 10.1021/bi00599a015. [DOI] [PubMed] [Google Scholar]
- Braiman M., Mathies R. Resonance Raman evidence for an all-trans to 13-cis isomerization in the proton-pumping cycle of bacteriorhodopsin. Biochemistry. 1980 Nov 11;19(23):5421–5428. doi: 10.1021/bi00564a042. [DOI] [PubMed] [Google Scholar]
- Draheim J. E., Cassim J. Y. Large Scale Global Structural Changes of the Purple Membrane during the Photocycle. Biophys J. 1985 Apr;47(4):497–507. doi: 10.1016/S0006-3495(85)83943-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenberg B., Berezin Y. Surface potential on purple membranes and its sidedness studied by a resonance Raman dye probe. Biophys J. 1984 Apr;45(4):663–670. doi: 10.1016/S0006-3495(84)84208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman D. M., Henderson R., McLachlan A. D., Wallace B. A. Path of the polypeptide in bacteriorhodopsin. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2023–2027. doi: 10.1073/pnas.77.4.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel R. D., Forsyth J. M. Time-resolved x-ray diffraction study of photostimulated purple membrane. Biophys J. 1985 Mar;47(3):387–393. doi: 10.1016/S0006-3495(85)83930-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaeser R. M., Jubb J. S., Henderson R. Structural comparison of native and deoxycholate-treated purple membrane. Biophys J. 1985 Nov;48(5):775–780. doi: 10.1016/S0006-3495(85)83835-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbison G. S., Smith S. O., Pardoen J. A., Winkel C., Lugtenburg J., Herzfeld J., Mathies R., Griffin R. G. Dark-adapted bacteriorhodopsin contains 13-cis, 15-syn and all-trans, 15-anti retinal Schiff bases. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1706–1709. doi: 10.1073/pnas.81.6.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward S. B., Glaeser R. M. Radiation damage of purple membrane at low temperature. Ultramicroscopy. 1979;04(2):201–210. doi: 10.1016/s0304-3991(79)90211-0. [DOI] [PubMed] [Google Scholar]
- Kalisky O., Ottolenghi M., Honig B., Korenstein R. Environmental effects on formation and photoreaction of the M412 photoproduct of bacteriorhodopsin: implications for the mechanism of proton pumping. Biochemistry. 1981 Feb 3;20(3):649–655. doi: 10.1021/bi00506a031. [DOI] [PubMed] [Google Scholar]
- Kimura Y., Fujiwara M., Ikegami A. Anisotropic electric properties of purple membrane and their change during the photoreaction cycle. Biophys J. 1984 Mar;45(3):615–625. doi: 10.1016/S0006-3495(84)84200-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenstein R., Hess B. Hydration effects on the photocycle of bacteriorhodopsin in thin layers of purple membrane. Nature. 1977 Nov 10;270(5633):184–186. doi: 10.1038/270184a0. [DOI] [PubMed] [Google Scholar]
- Lazarev Y. A., Terpugov E. L. Effect of water on the structure of bacteriorhodopsin and photochemical processes in purple membranes. Biochim Biophys Acta. 1980 May 9;590(3):324–338. doi: 10.1016/0005-2728(80)90203-0. [DOI] [PubMed] [Google Scholar]
- Lewis A., Spoonhower J., Bogomolni R. A., Lozier R. H., Stoeckenius W. Tunable laser resonance raman spectroscopy of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4462–4466. doi: 10.1073/pnas.71.11.4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozier R. H., Niederberger W. The photochemical cycle of bacteriorhodopsin. Fed Proc. 1977 May;36(6):1805–1809. [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Functions of a new photoreceptor membrane. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2853–2857. doi: 10.1073/pnas.70.10.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Rhodopsin-like protein from the purple membrane of Halobacterium halobium. Nat New Biol. 1971 Sep 29;233(39):149–152. doi: 10.1038/newbio233149a0. [DOI] [PubMed] [Google Scholar]
- Ort D. R., Parson W. W. Enthalpy changes during the photochemical cycle of bacteriorhodopsin. Biophys J. 1979 Feb;25(2 Pt 1):355–364. doi: 10.1016/s0006-3495(79)85297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renthal R., Cha C. H. Charge asymmetry of the purple membrane measured by uranyl quenching of dansyl fluorescence. Biophys J. 1984 May;45(5):1001–1006. doi: 10.1016/S0006-3495(84)84245-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothschild K. J., Zagaeski M., Cantore W. A. Conformational changes of bacteriorhodopsin detected by Fourier transform infrared difference spectroscopy. Biochem Biophys Res Commun. 1981 Nov 30;103(2):483–489. doi: 10.1016/0006-291x(81)90478-2. [DOI] [PubMed] [Google Scholar]
- Sturtevant J. M. Heat capacity and entropy changes in processes involving proteins. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2236–2240. doi: 10.1073/pnas.74.6.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M., Govindjee R., Ebrey T. G. Effects of pressure and temperature on the M412 intermediate of the bacteriorhodopsin photocycle. Implications for the phase transition of the purple membrane. Biophys J. 1983 Nov;44(2):249–254. doi: 10.1016/S0006-3495(83)84296-9. [DOI] [PMC free article] [PubMed] [Google Scholar]



