Abstract
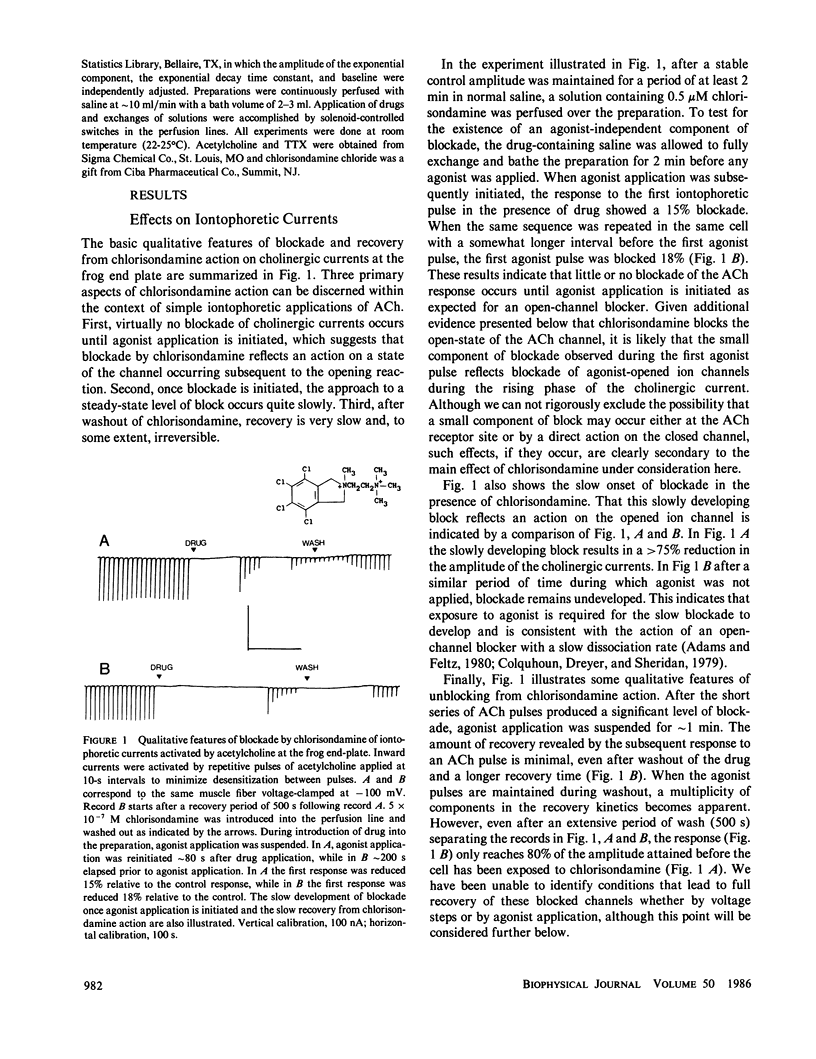
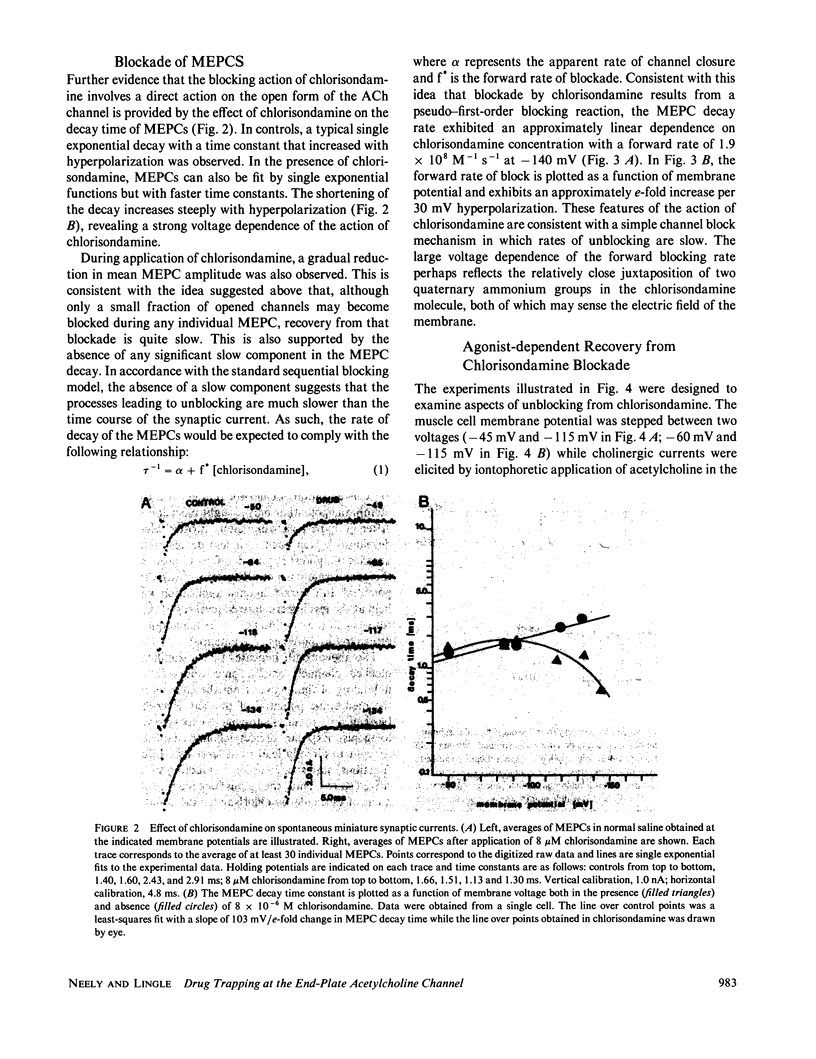
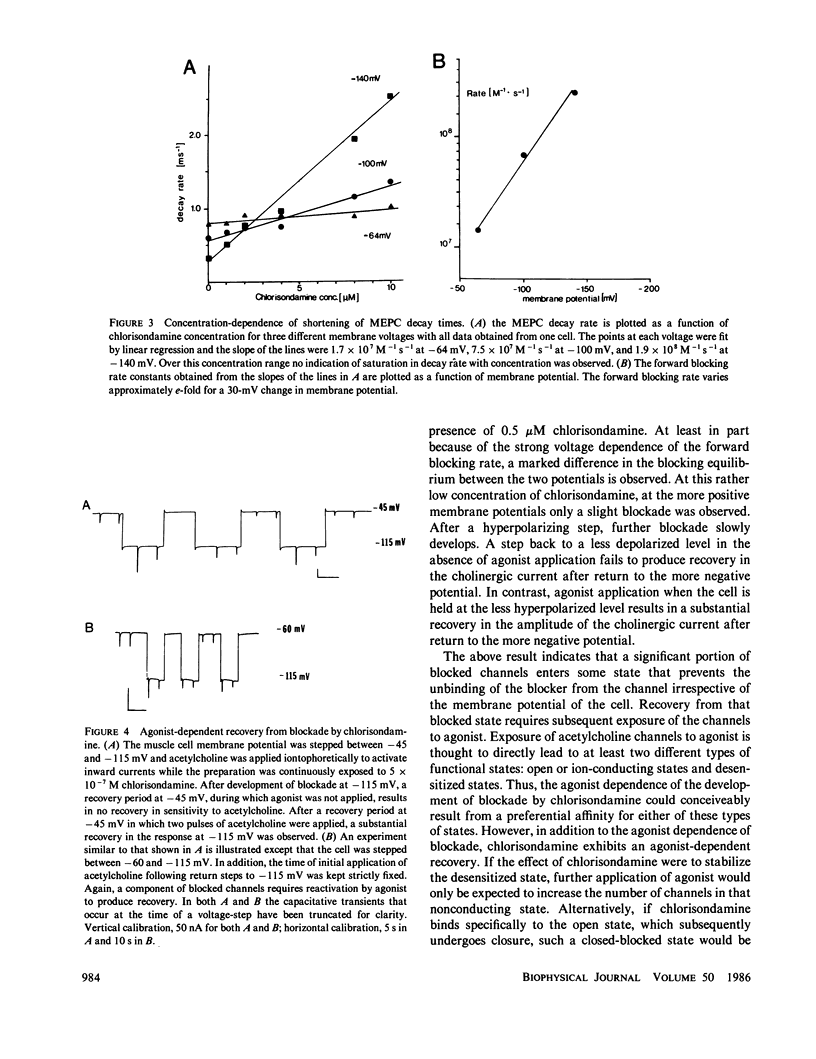
At the ganglionic nicotinic acetylcholine channel (Gurney, A. M., and H. P. Rang, 1984, Br. J. Pharmacol., 82:623-642) and on some cholinergic neuromuscular synapses of Crustacea (Lingle, C., 1983a, J. Physiol. (Lond.), 339:395-417; Lingle, C., 1983b, J. Physiol. (Lond.), 339:419-437), some agents that block cholinergic currents by an open-channel block mechanism appear to become trapped within the channel when it subsequently closes. It is unknown whether trapping of some open-channel blockers might also occur at the neuromuscular nicotinic acetylcholine channel. Here we show that the long-lived cholinergic blocking action of chlorisondamine, a ganglionic nicotinic blocker, can in part be most simply explained by an open-channel block mechanism followed by a subsequent trapping of the blocking molecule within the closed ion channel. Unique structural characteristics of the chlorisondamine molecule place several provocative constraints on the mechanism by which trapping may be occurring.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R. A study of desensitization using voltage clamp. Pflugers Arch. 1975 Oct 28;360(2):135–144. doi: 10.1007/BF00580536. [DOI] [PubMed] [Google Scholar]
- Adams P. R., Feltz A. Quinacrine (mepacrine) action at frog end-plate. J Physiol. 1980 Sep;306:261–281. doi: 10.1113/jphysiol.1980.sp013396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R., Sakmann B. Decamethonium both opens and blocks endplate channels. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2994–2998. doi: 10.1073/pnas.75.6.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R. Voltage jump analysis of procaine action at frog end-plate. J Physiol. 1977 Jun;268(2):291–318. doi: 10.1113/jphysiol.1977.sp011858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M. Interaction of tetraethylammonium ion derivatives with the potassium channels of giant axons. J Gen Physiol. 1971 Oct;58(4):413–437. doi: 10.1085/jgp.58.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisson A., Unwin P. N. Quaternary structure of the acetylcholine receptor. Nature. 1985 Jun 6;315(6019):474–477. doi: 10.1038/315474a0. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Dreyer F., Sheridan R. E. The actions of tubocurarine at the frog neuromuscular junction. J Physiol. 1979 Aug;293:247–284. doi: 10.1113/jphysiol.1979.sp012888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creese R., England J. M. Decamethonium in depolarized muscle and the effects of tubocurarine. J Physiol. 1970 Sep;210(2):345–361. doi: 10.1113/jphysiol.1970.sp009214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer T. M., Adams D. J., Hille B. The permeability of the endplate channel to organic cations in frog muscle. J Gen Physiol. 1980 May;75(5):469–492. doi: 10.1085/jgp.75.5.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., Wachtel R. E. Some effects of procaine at the toad end-plate are not consistent with a simple channel-blocking model. J Physiol. 1984 Jan;346:331–339. doi: 10.1113/jphysiol.1984.sp015025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney A. M., Rang H. P. The channel-blocking action of methonium compounds on rat submandibular ganglion cells. Br J Pharmacol. 1984 Jul;82(3):623–642. doi: 10.1111/j.1476-5381.1984.tb10801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Local anesthetics: hydrophilic and hydrophobic pathways for the drug-receptor reaction. J Gen Physiol. 1977 Apr;69(4):497–515. doi: 10.1085/jgp.69.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingle C. Blockade of cholinergic channels by chlorisondamine on a crustacean muscle. J Physiol. 1983 Jun;339:395–417. doi: 10.1113/jphysiol.1983.sp014723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingle C. Different types of blockade of crustacean acetylcholine-induced currents. J Physiol. 1983 Jun;339:419–437. doi: 10.1113/jphysiol.1983.sp014724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. Bis-quaternary ammonium blockers as structural probes of the sarcoplasmic reticulum K+ channel. J Gen Physiol. 1982 May;79(5):869–891. doi: 10.1085/jgp.79.5.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne R. J., Byrne J. H. Effects of hexamethonium and decamethonium on end-plate current parameters. Mol Pharmacol. 1981 Mar;19(2):276–281. [PubMed] [Google Scholar]
- Neher E. The charge carried by single-channel currents of rat cultured muscle cells in the presence of local anaesthetics. J Physiol. 1983 Jun;339:663–678. doi: 10.1113/jphysiol.1983.sp014741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varanda W. A., Aracava Y., Sherby S. M., VanMeter W. G., Eldefrawi M. E., Albuquerque E. X. The acetylcholine receptor of the neuromuscular junction recognizes mecamylamine as a noncompetitive antagonist. Mol Pharmacol. 1985 Aug;28(2):128–137. [PubMed] [Google Scholar]
- Wachtel R. E., Wilson W. A., Jr Barbiturate effects on acetylcholine-activated channels in Aplysia neurons. Mol Pharmacol. 1983 Nov;24(3):449–457. [PubMed] [Google Scholar]


