Abstract
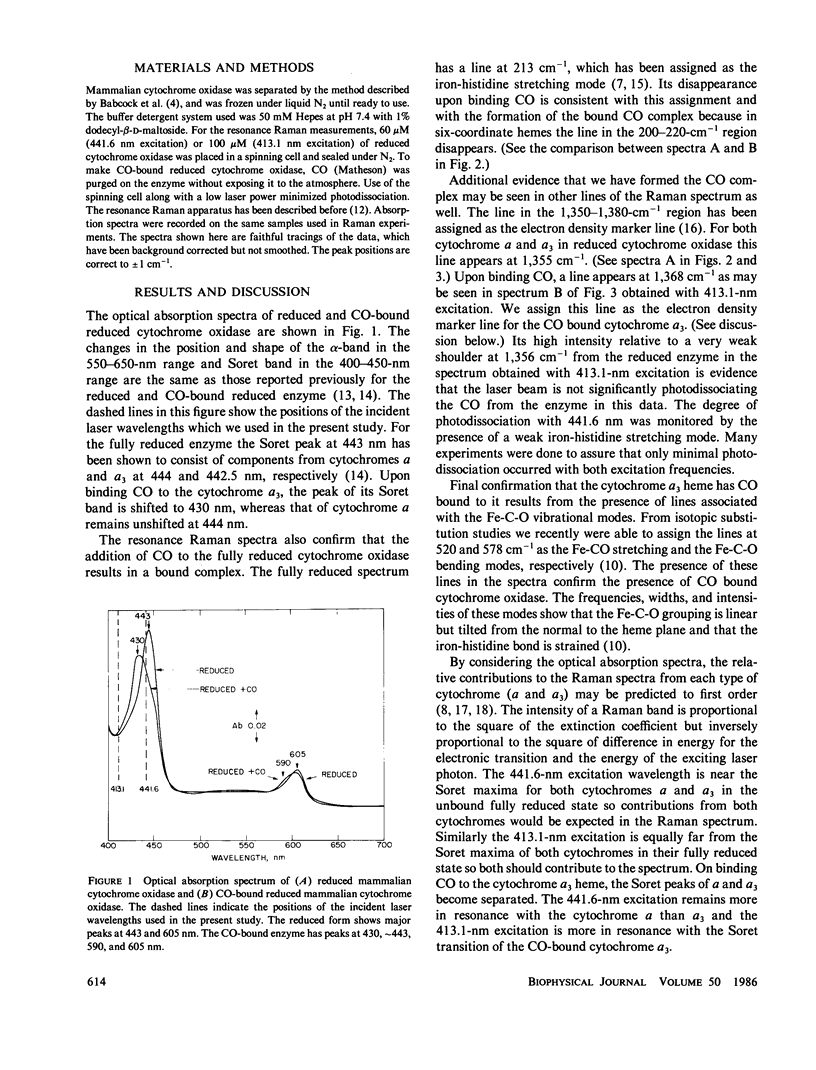
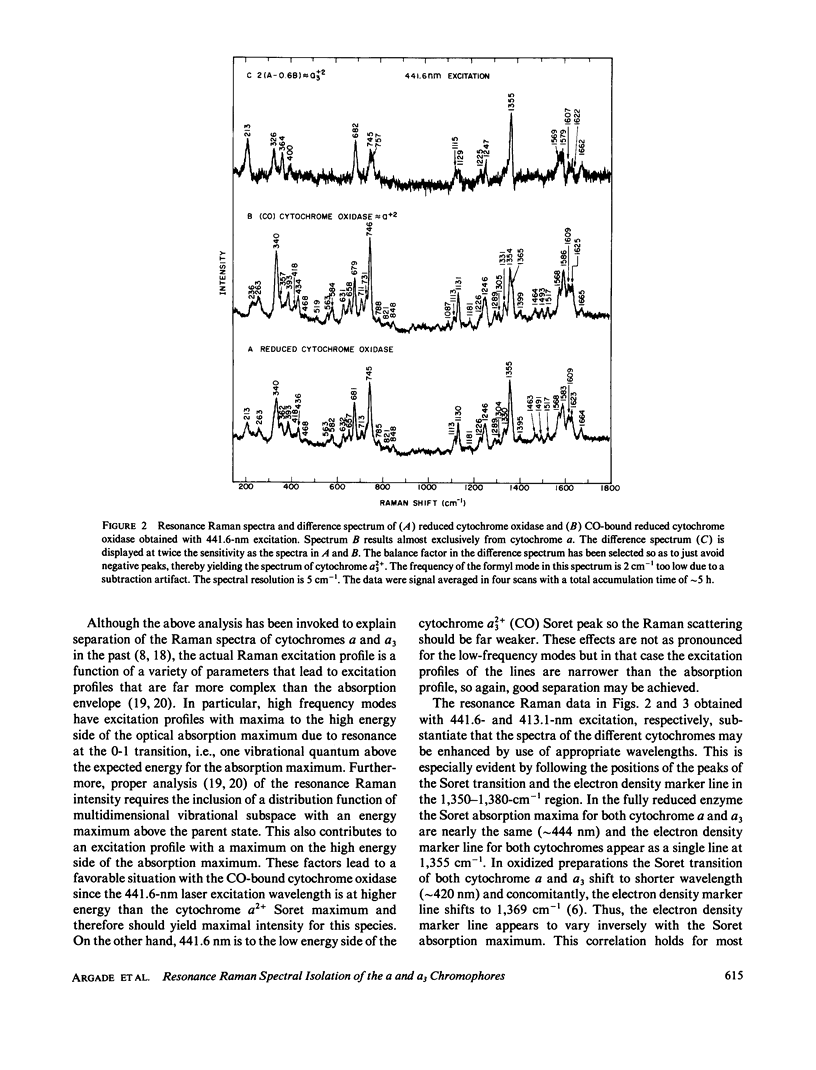
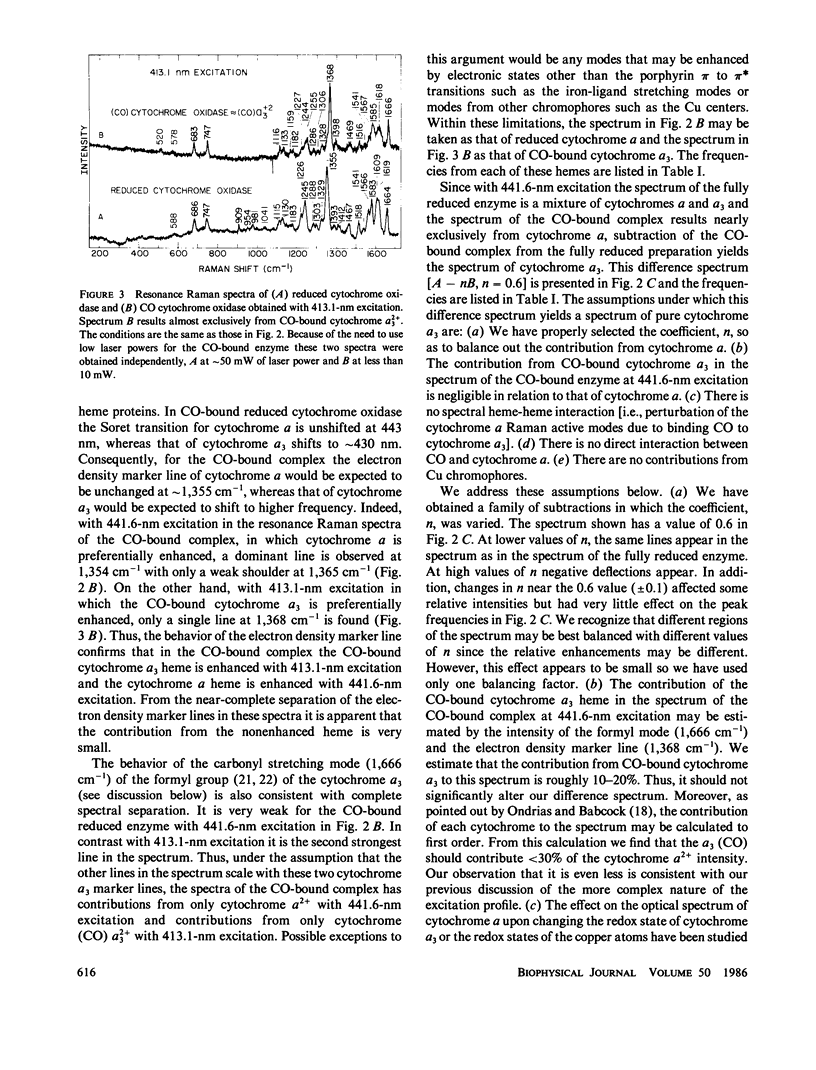
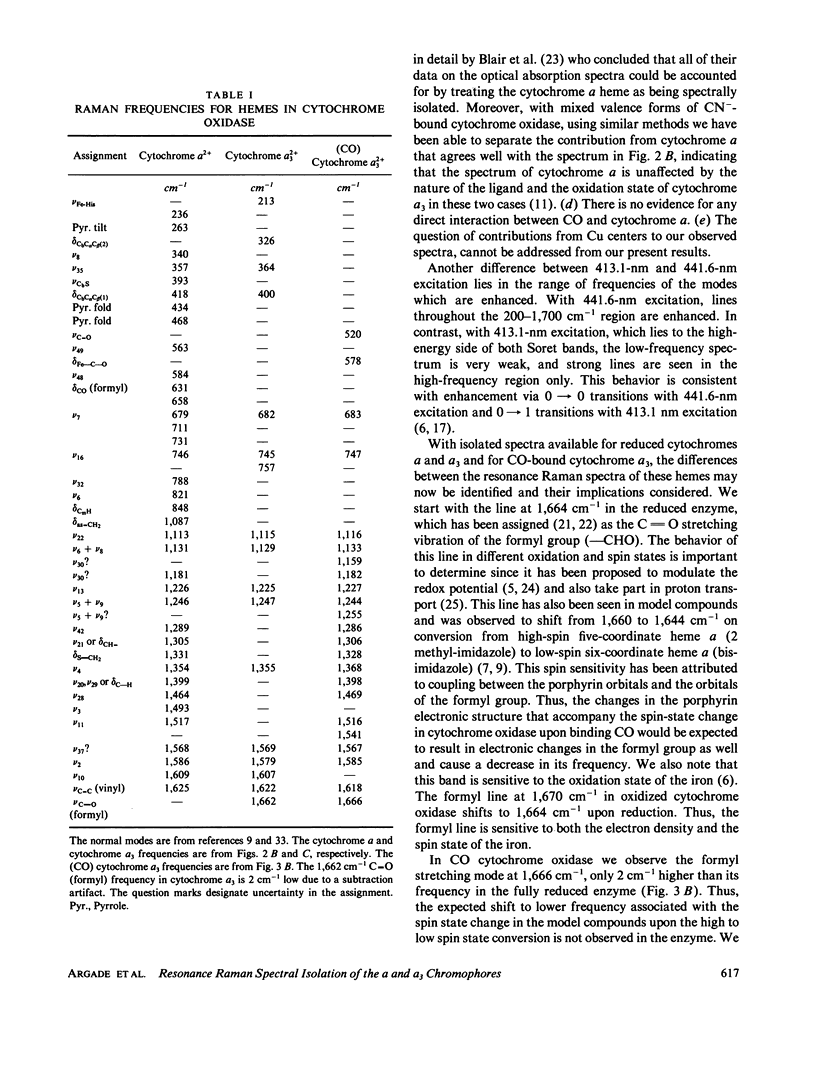
Resonance Raman spectra of reduced CO-bound cytochrome oxidase obtained at two different excitation frequencies (441.6 and 413.1 nm) are compared with the spectra of the fully reduced enzyme. In the spectra of the CO-bound complex only the cytochrome a modes are strongly enhanced with 441.6 nm excitation and only the modes of the CO-bound cytochrome a3 heme are strongly enhanced with 413.1-nm excitation. In the fully reduced complex with both excitation frequencies, modes of both cytochrome a and a3 are enhanced. By subtraction we are able to uncover the complete spectrum of the fully reduced ligand-free cytochrome a3 heme. Thus, we report the discrete resonance Raman spectra of cytochromes a2+, a2+3, and a2+3 (CO). The spectra of fully reduced cytochrome a and ligand-free cytochrome a3 are very different especially in the low frequency region. Binding CO to ferrous cytochrome a3 results in electronic structure changes in the heme analogous to those in hemoglobin and myoglobin, from which we conclude that there is nothing electronically unique in the ferrous cytochrome a3 heme to account for its catalytic properties.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argade P. V., Ching Y. C., Rousseau D. L. Cytochrome a3 structure in carbon monoxide-bound cytochrome oxidase. Science. 1984 Jul 20;225(4659):329–331. doi: 10.1126/science.6330890. [DOI] [PubMed] [Google Scholar]
- Babcock G. T., Callahan P. M., Ondrias M. R., Salmeen I. Coordination geometries and vibrational properties of cytochromes alpha and alpha 3 in cytochrome oxidase from Soret excitation Raman spectroscopy. Biochemistry. 1981 Feb 17;20(4):959–966. doi: 10.1021/bi00507a049. [DOI] [PubMed] [Google Scholar]
- Babcock G. T., Callahan P. M. Redox-linked hydrogen bond strength changes in cytochrome a: implications for a cytochrome oxidase proton pump. Biochemistry. 1983 May 10;22(10):2314–2319. doi: 10.1021/bi00279a002. [DOI] [PubMed] [Google Scholar]
- Babcock G. T., Salmeen I. Resonance Raman spectra and optical properties of oxidized cytochrome oxidase. Biochemistry. 1979 Jun 12;18(12):2493–2498. doi: 10.1021/bi00579a009. [DOI] [PubMed] [Google Scholar]
- Babcock G. T., Vickery L. E., Palmer G. Electronic state of heme in cytochrome oxidase. I. Magnetic circular dichroism of the isolated enzyme and its derivatives. J Biol Chem. 1976 Dec 25;251(24):7907–7919. [PubMed] [Google Scholar]
- Blair D. F., Bocian D. F., Babcock G. T., Chan S. I. Evidence for modulation of the heme absorptions of cytochrome c oxidase by metal-metal interactions. Biochemistry. 1982 Dec 21;21(26):6928–6935. doi: 10.1021/bi00269a048. [DOI] [PubMed] [Google Scholar]
- Callahan P. M., Babcock G. T. Insights into heme structure from Soret excitation Raman spectroscopy. Biochemistry. 1981 Feb 17;20(4):952–958. doi: 10.1021/bi00507a048. [DOI] [PubMed] [Google Scholar]
- Callahan P. M., Babcock G. T. Origin of the cytochrome a absorption red shift: a pH-dependent interaction between its heme a formyl and protein in cytochrome oxidase. Biochemistry. 1983 Jan 18;22(2):452–461. doi: 10.1021/bi00271a031. [DOI] [PubMed] [Google Scholar]
- Chance B., Saronio C., Leigh J. S., Jr Functional intermediates in the reaction of membrane-bound cytochrome oxidase with oxygen. J Biol Chem. 1975 Dec 25;250(24):9226–9237. [PubMed] [Google Scholar]
- Ching Y. C., Argade P. V., Rousseau D. L. Resonance raman spectra of CN--bound cytochrome oxidase: spectral isolation of cytochromes a2+, a3(2+), and a3(2+)(CN-). Biochemistry. 1985 Aug 27;24(18):4938–4946. doi: 10.1021/bi00339a032. [DOI] [PubMed] [Google Scholar]
- Ondrias M. R., Babcock G. T. Resonance enhancement of the vibrations of cytochrome -a3 and its conformation in oxidized cytochrome oxidase. Biochem Biophys Res Commun. 1980 Mar 13;93(1):29–35. doi: 10.1016/s0006-291x(80)80241-5. [DOI] [PubMed] [Google Scholar]
- Ondrias M. R., Rousseau D. L., Simon S. R. Resonance Raman spectra of photodissociated carbonmonoxy hemoglobin and deoxy hemoglobin at 10 K. J Biol Chem. 1983 May 10;258(9):5638–5642. [PubMed] [Google Scholar]
- Rousseau D. L., Ondrias M. R., LaMar G. N., Kong S. B., Smith K. M. Resonance Raman spectra of the heme in leghemoglobin. Evidence for the absence of ruffling and the influence of the vinyl groups. J Biol Chem. 1983 Feb 10;258(3):1740–1746. [PubMed] [Google Scholar]
- Salmeen I., Rimai L., Babcock G. Raman spectra of heme a, cytochrome oxidase-ligand complexes, and alkaline denatured oxidase. Biochemistry. 1978 Mar 7;17(5):800–806. doi: 10.1021/bi00598a008. [DOI] [PubMed] [Google Scholar]
- Spiro T. G., Strekas T. C. Resonance Raman spectra of heme proteins. Effects of oxidation and spin state. J Am Chem Soc. 1974 Jan 23;96(2):338–345. doi: 10.1021/ja00809a004. [DOI] [PubMed] [Google Scholar]
- Tsubaki M., Srivastava R. B., Yu N. T. Resonance Raman investigation of carbon monoxide bonding in (carbon monoxy)hemoglobin and -myoglobin: detection of Fe-CO stretching and Fe-C-O bending vibrations and influence of the quaternary structure change. Biochemistry. 1982 Mar 16;21(6):1132–1140. doi: 10.1021/bi00535a004. [DOI] [PubMed] [Google Scholar]
- Vanneste W. H. The stoichiometry and absorption spectra of components a and a-3 in cytochrome c oxidase. Biochemistry. 1966 Mar;5(3):838–848. doi: 10.1021/bi00867a005. [DOI] [PubMed] [Google Scholar]
- Woodruff W. H., Dallinger R. F., Antalis T. M., Palmer G. Resonance Raman spectroscopy of cytochrome oxidase using Soret excitation: selective enhancement, indicator bands, and structural significance for cytochromes a and a3. Biochemistry. 1981 Mar 3;20(5):1332–1338. doi: 10.1021/bi00508a045. [DOI] [PubMed] [Google Scholar]
- Yoshikawa S., Choc M. G., O'Toole M. C., Caughey W. S. An infrared study of CO binding to heart cytochrome c oxidase and hemoglobin A. Implications re O2 reactions. J Biol Chem. 1977 Aug 10;252(15):5498–5508. [PubMed] [Google Scholar]


