Abstract
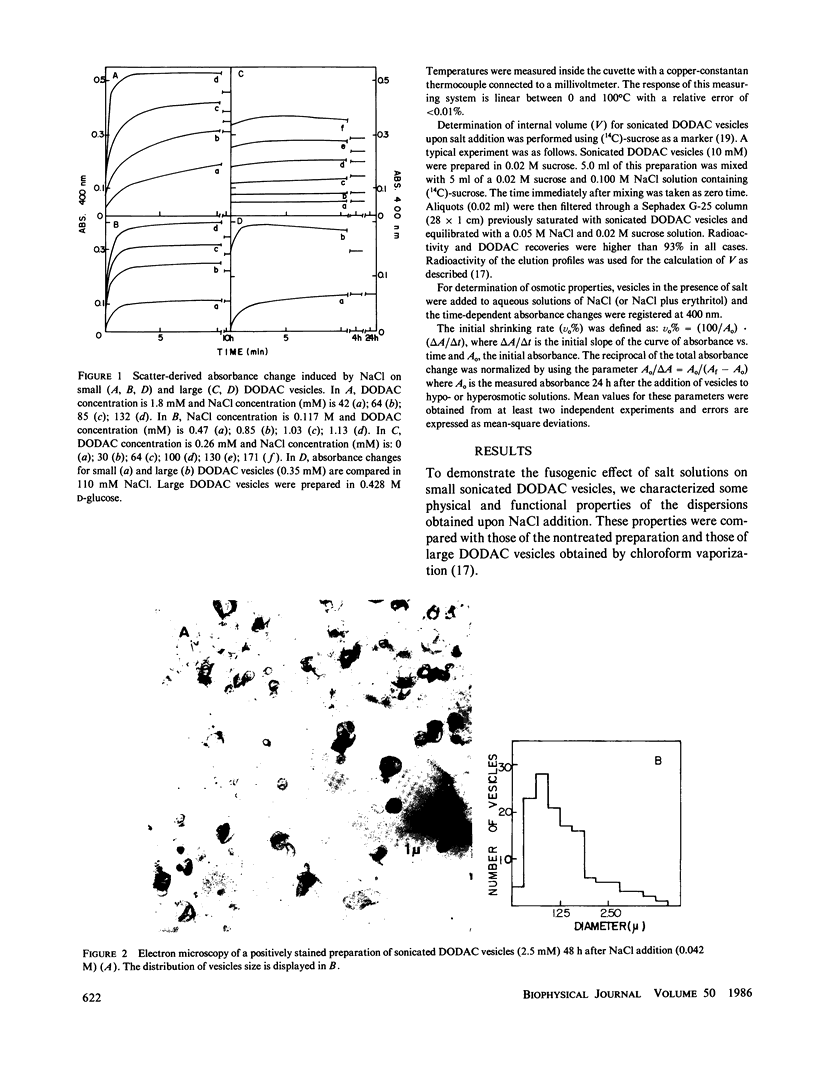
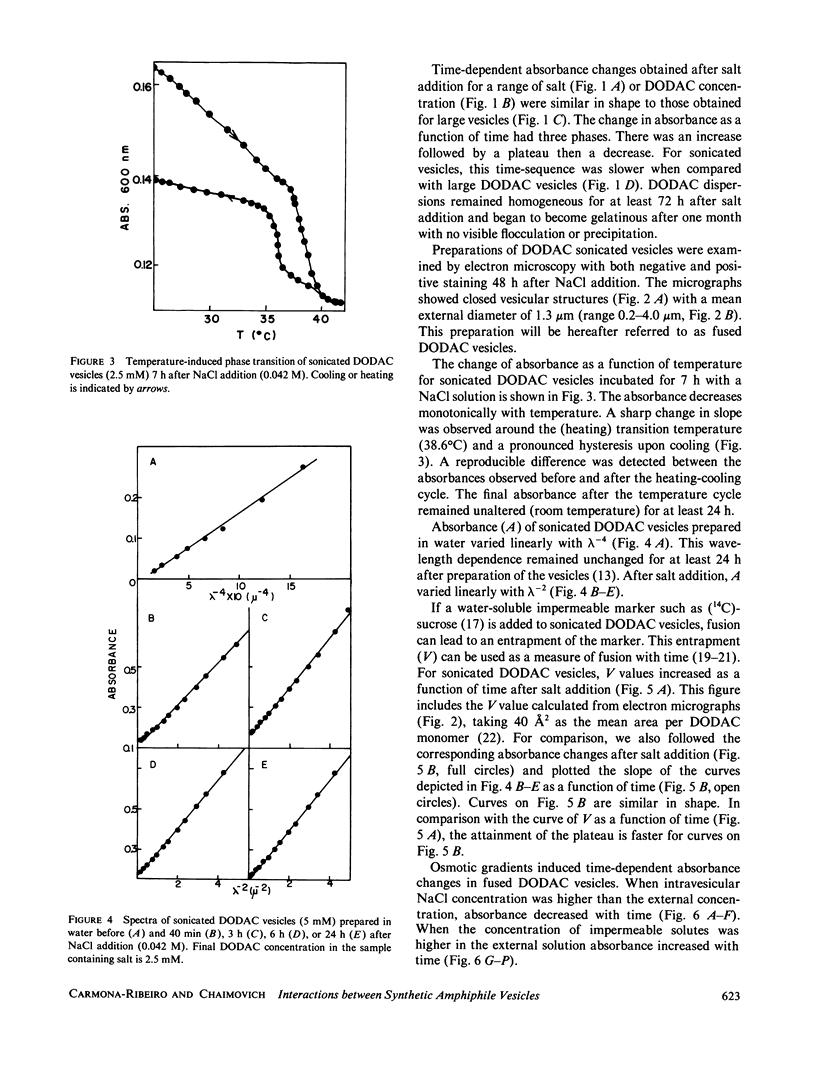
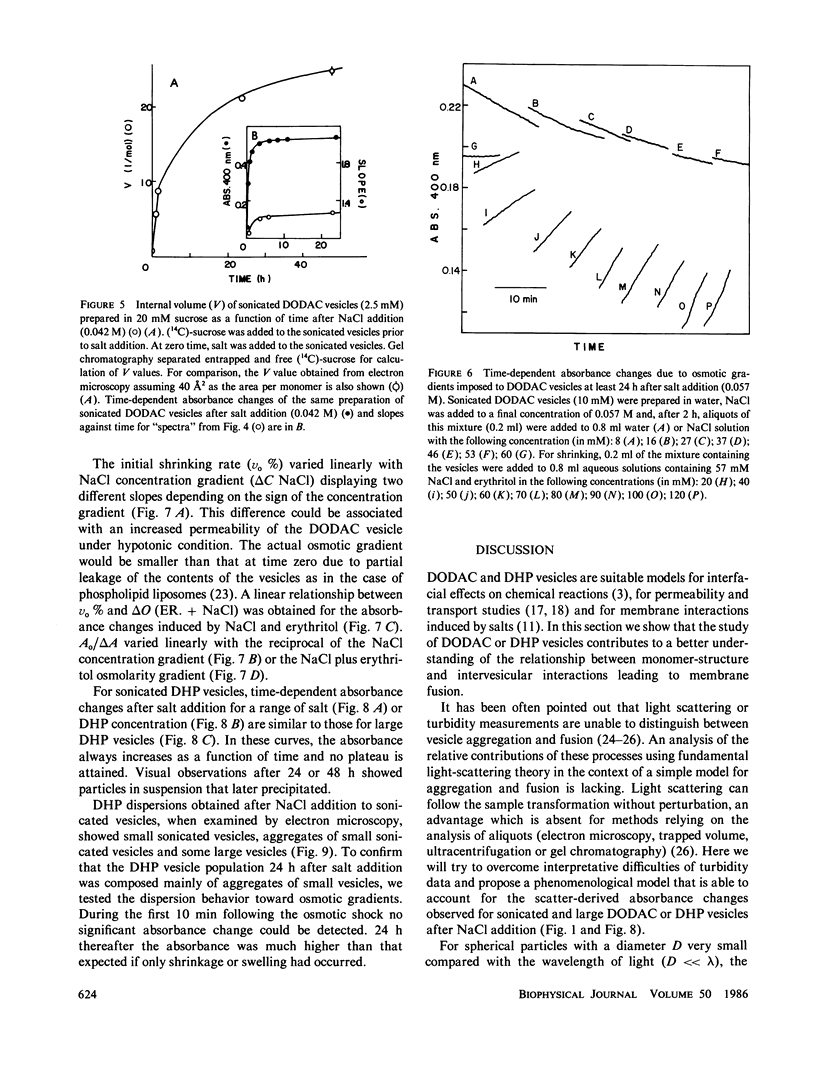
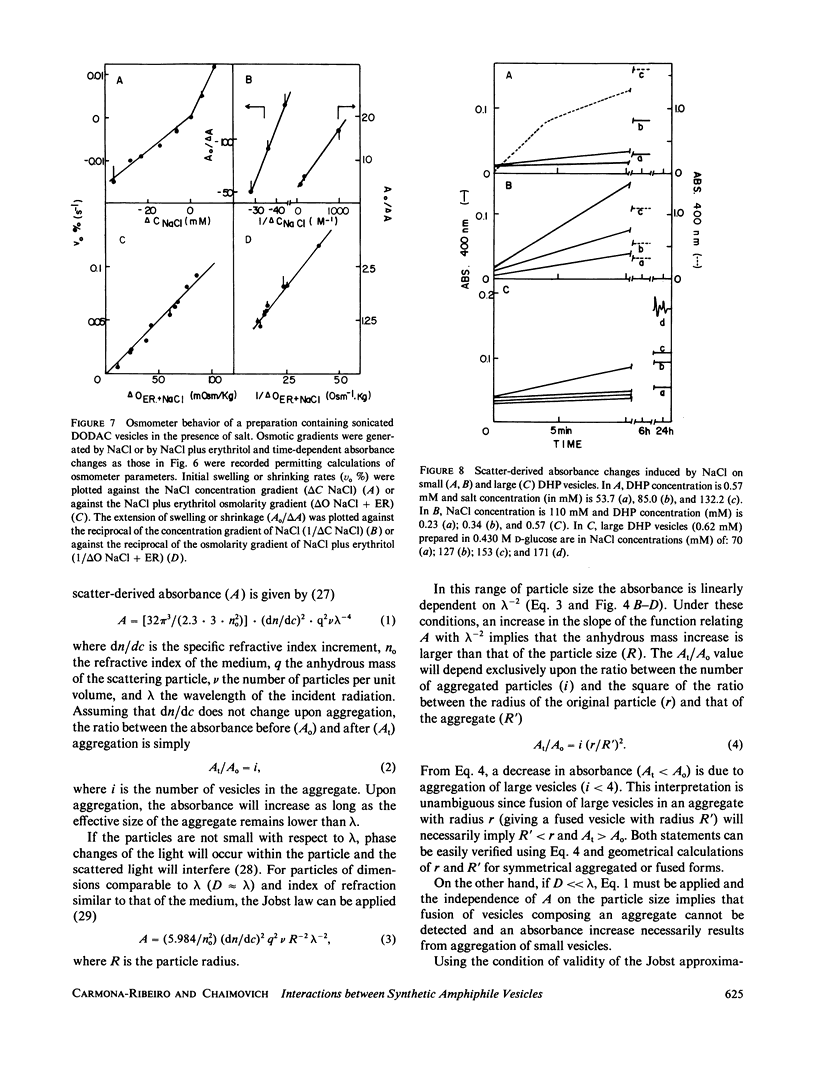
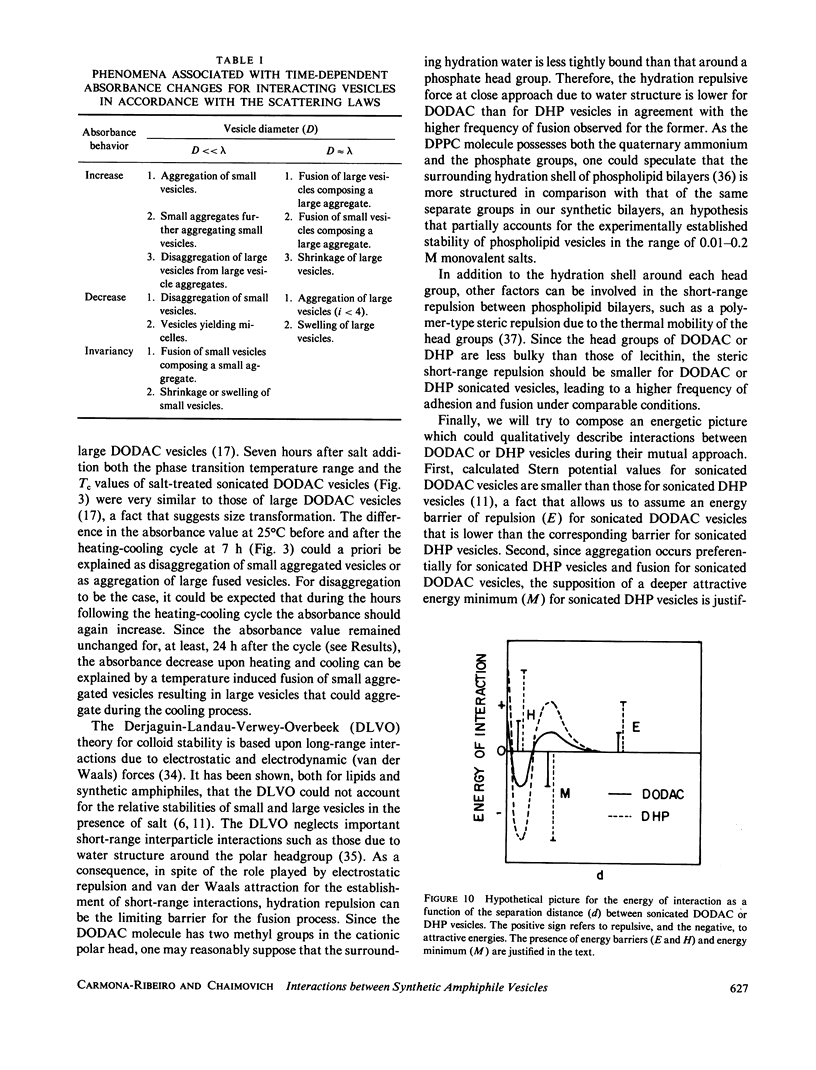
Small dioctadecyldimethylammonium chloride (DODAC) vesicles prepared by sonication fuse upon addition of NaCl as detected by several methods (electron microscopy, trapped volume determinations, temperature-dependent phase transition curves, and osmometer behavior. In contrast, small sodium dihexadecyl phosphate (DHP) vesicles mainly aggregate upon NaCl addition as shown by electron microscopy and the lack of osmometer behavior. Scatter-derived absorbance changes of small and large DODAC or DHP vesicles as a function of time after salt addition were obtained for a range of NaCl or amphiphile concentration. These changes were interpreted in accordance with a phenomenological model based upon fundamental light-scattering laws and simple geometrical considerations. Short-range hydration repulsion between DODAC (or DHP) vesicles is possibly the main energy barrier for the fusion process.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chong C. S., Colbow K. Light scattering and turbidity measurements on lipid vesicles. Biochim Biophys Acta. 1976 Jun 17;436(2):260–282. doi: 10.1016/0005-2736(76)90192-9. [DOI] [PubMed] [Google Scholar]
- Dimitrov D. S., Zhelev D. V., Jain R. K. Stability of membrane systems modeled as multilayered viscoelastic films. J Theor Biol. 1985 Mar 21;113(2):353–377. doi: 10.1016/s0022-5193(85)80232-0. [DOI] [PubMed] [Google Scholar]
- Johnson S. M., Buttress N. The osmotic insensitivity of sonicated liposomes and the density of phospholipid-cholesterol mixtures. Biochim Biophys Acta. 1973 Apr 25;307(1):20–26. doi: 10.1016/0005-2736(73)90021-7. [DOI] [PubMed] [Google Scholar]
- KOCH A. L. Some calculations on the turbidity of mitochondria and bacteria. Biochim Biophys Acta. 1961 Aug 19;51:429–441. doi: 10.1016/0006-3002(61)90599-6. [DOI] [PubMed] [Google Scholar]
- LeNeveu D. M., Rand R. P. Measurement and modification of forces between lecithin bilayers. Biophys J. 1977 May;18(2):209–230. doi: 10.1016/S0006-3495(77)85608-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marra J., Israelachvili J. Direct measurements of forces between phosphatidylcholine and phosphatidylethanolamine bilayers in aqueous electrolyte solutions. Biochemistry. 1985 Aug 13;24(17):4608–4618. doi: 10.1021/bi00338a020. [DOI] [PubMed] [Google Scholar]
- Morgan C. G., Williamson H., Fuller S., Hudson B. Melittin induces fusion of unilamellar phospholipid vesicles. Biochim Biophys Acta. 1983 Aug 10;732(3):668–674. doi: 10.1016/0005-2736(83)90245-6. [DOI] [PubMed] [Google Scholar]
- Mortara R. A., Quina F. H., Chaimovich H. Formation of closed vesicles from a simple phosphate diester. Preparation and some properties of vesicles of dihexadecyl phosphate. Biochem Biophys Res Commun. 1978 Apr 28;81(4):1080–1086. doi: 10.1016/0006-291x(78)91246-9. [DOI] [PubMed] [Google Scholar]
- Ohki S., Düzgüneş N., Leonards K. Phospholipid vesicle aggregation: effect of monovalent and divalent ions. Biochemistry. 1982 Apr 27;21(9):2127–2133. doi: 10.1021/bi00538a022. [DOI] [PubMed] [Google Scholar]
- Ohki S., Roy S., Ohshima H., Leonards K. Monovalent cation-induced phospholipid vesicle aggregation: effect of ion binding. Biochemistry. 1984 Dec 4;23(25):6126–6132. doi: 10.1021/bi00320a035. [DOI] [PubMed] [Google Scholar]
- Ribeiro A. M., Chaimovich H. Preparation and characterization of large dioctadecyldimethylammonium chloride liposomes and comparison with small sonicated vesicles. Biochim Biophys Acta. 1983 Aug 24;733(1):172–179. doi: 10.1016/0005-2736(83)90103-7. [DOI] [PubMed] [Google Scholar]
- Rouser G., Fkeischer S., Yamamoto A. Two dimensional then layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids. 1970 May;5(5):494–496. doi: 10.1007/BF02531316. [DOI] [PubMed] [Google Scholar]
- Schenkman S., Araujo P. S., Dijkman R., Quina F. H., Chaimovich H. Effects of temperature and lipid composition on the serum albumin-induced aggregation and fusion of small unilamellar vesicles. Biochim Biophys Acta. 1981 Dec 21;649(3):633–647. doi: 10.1016/0005-2736(81)90168-1. [DOI] [PubMed] [Google Scholar]
- Schmidt C. F., Lichtenberg D., Thompson T. E. Vesicle- vesicle interactions in sonicated dispersions of dipalmitoylphosphatidylcholine. Biochemistry. 1981 Aug 4;20(16):4792–4797. doi: 10.1021/bi00519a041. [DOI] [PubMed] [Google Scholar]
- Wilschut J., Papahadjopoulos D. Ca2+-induced fusion of phospholipid vesicles monitored by mixing of aqueous contents. Nature. 1979 Oct 25;281(5733):690–692. doi: 10.1038/281690a0. [DOI] [PubMed] [Google Scholar]
- Wong M., Thompson T. E. Aggregation of dipalmitoylphosphatidylcholine vesicles. Biochemistry. 1982 Aug 17;21(17):4133–4139. doi: 10.1021/bi00260a033. [DOI] [PubMed] [Google Scholar]




