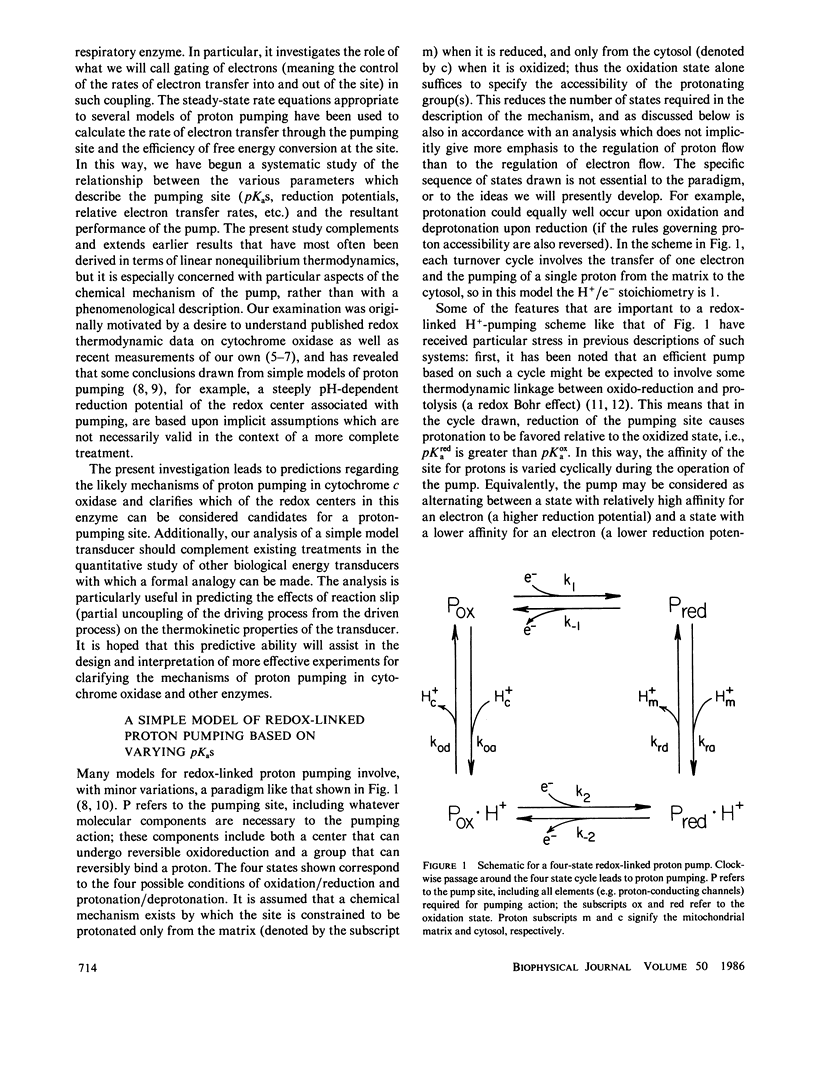
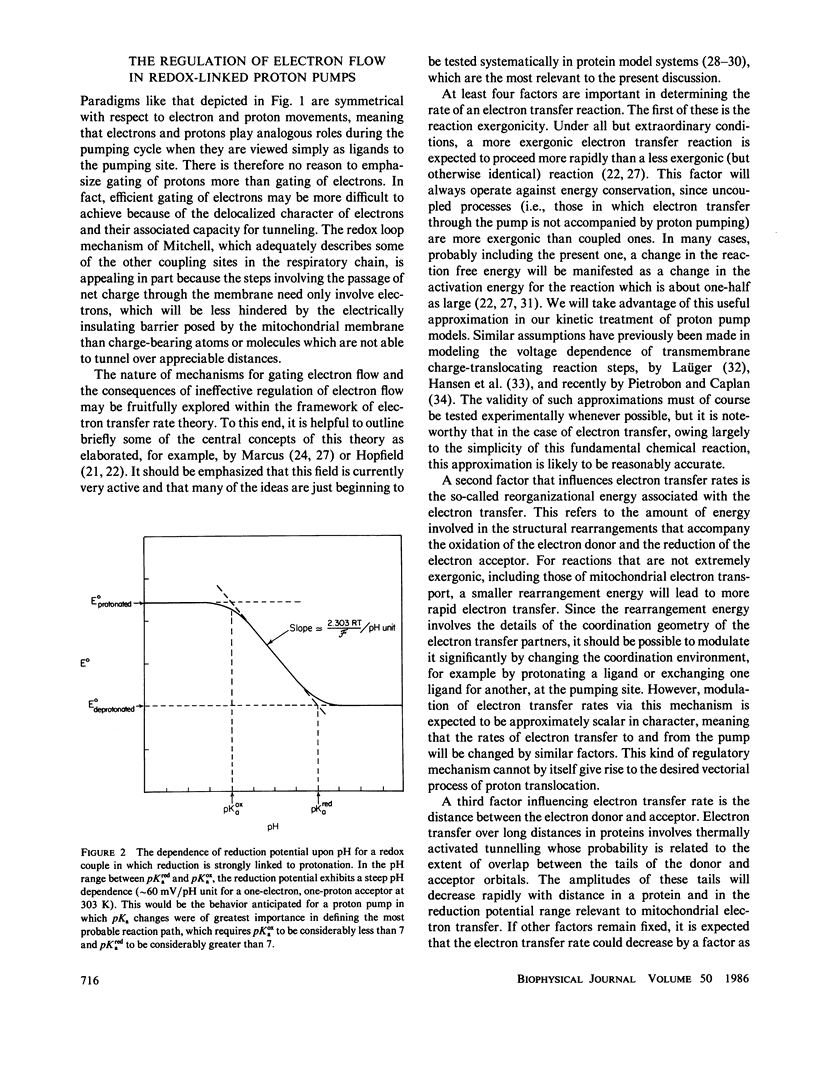
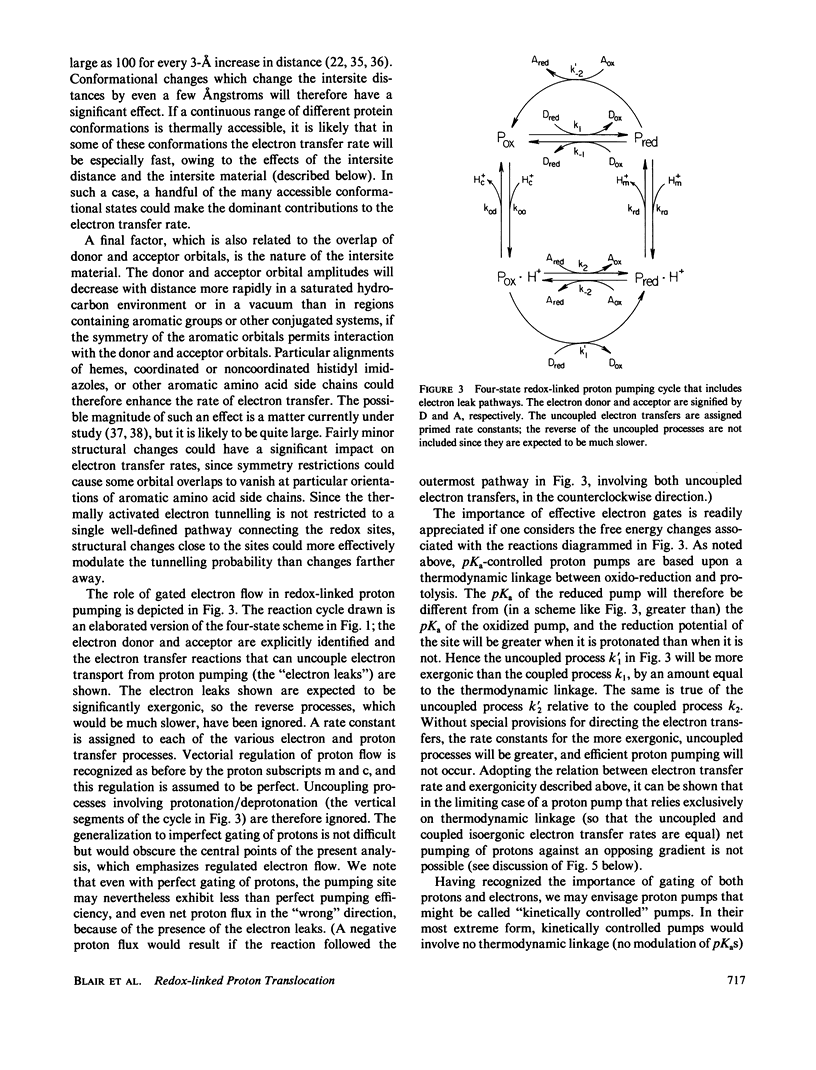
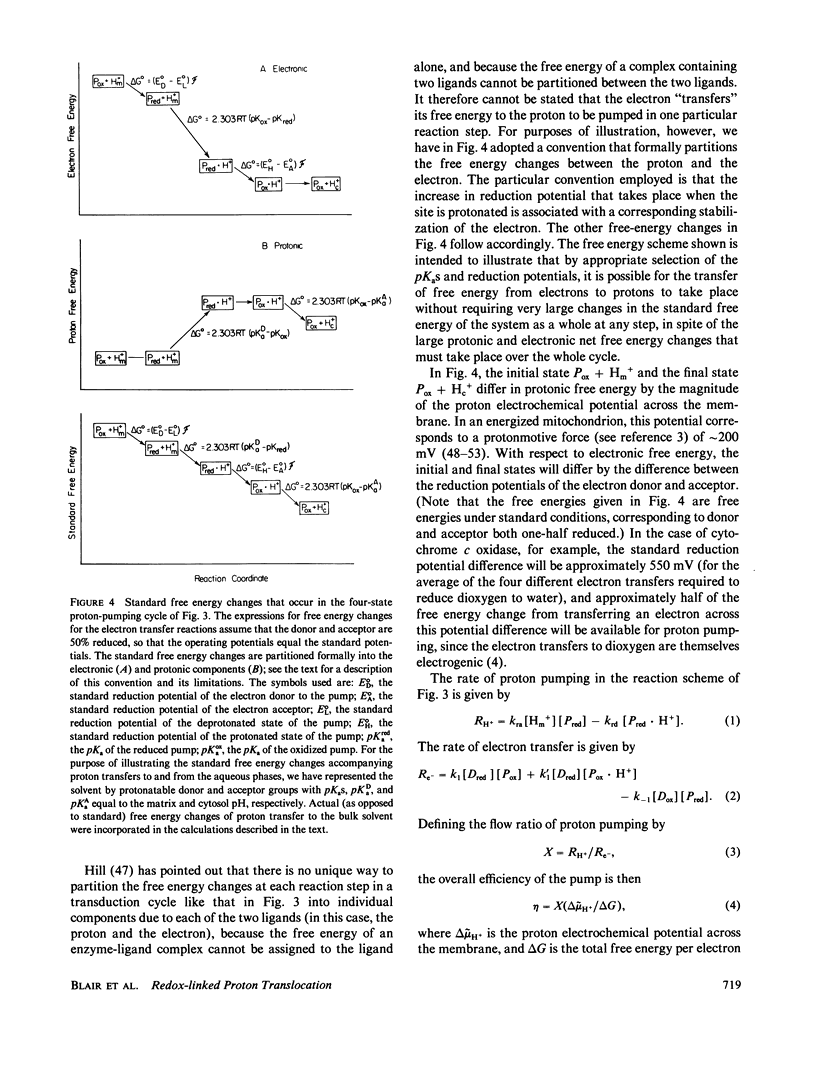
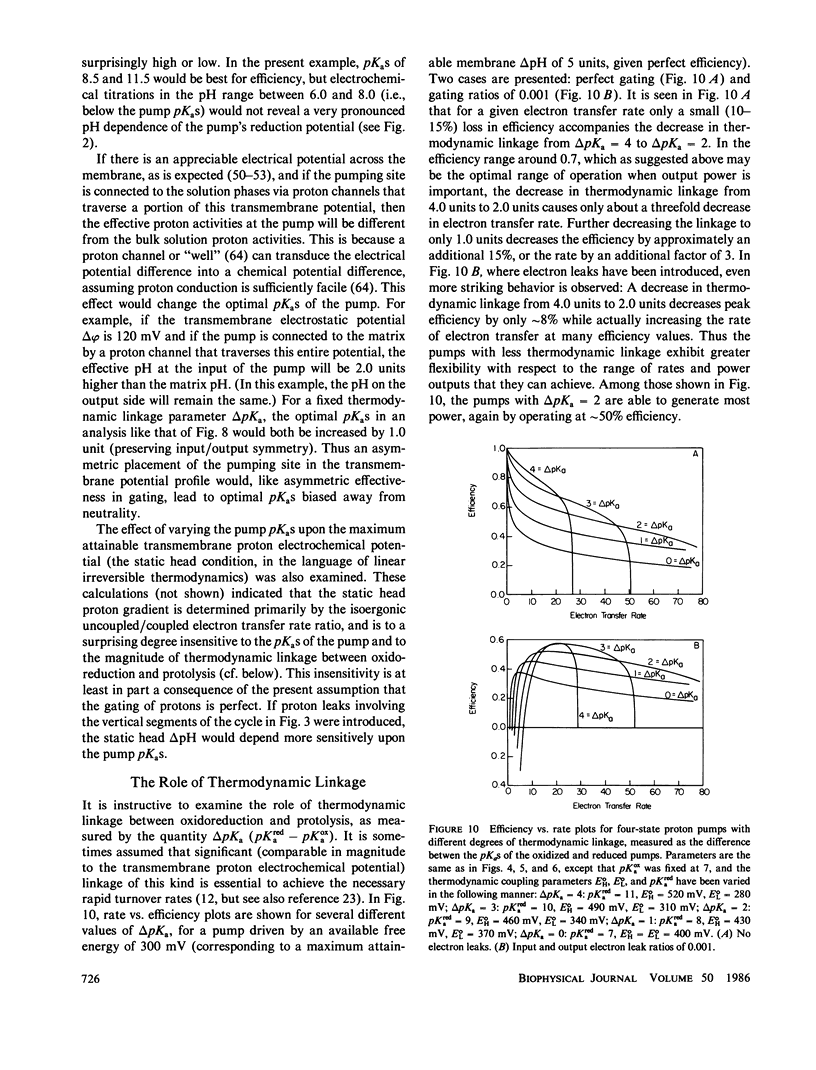
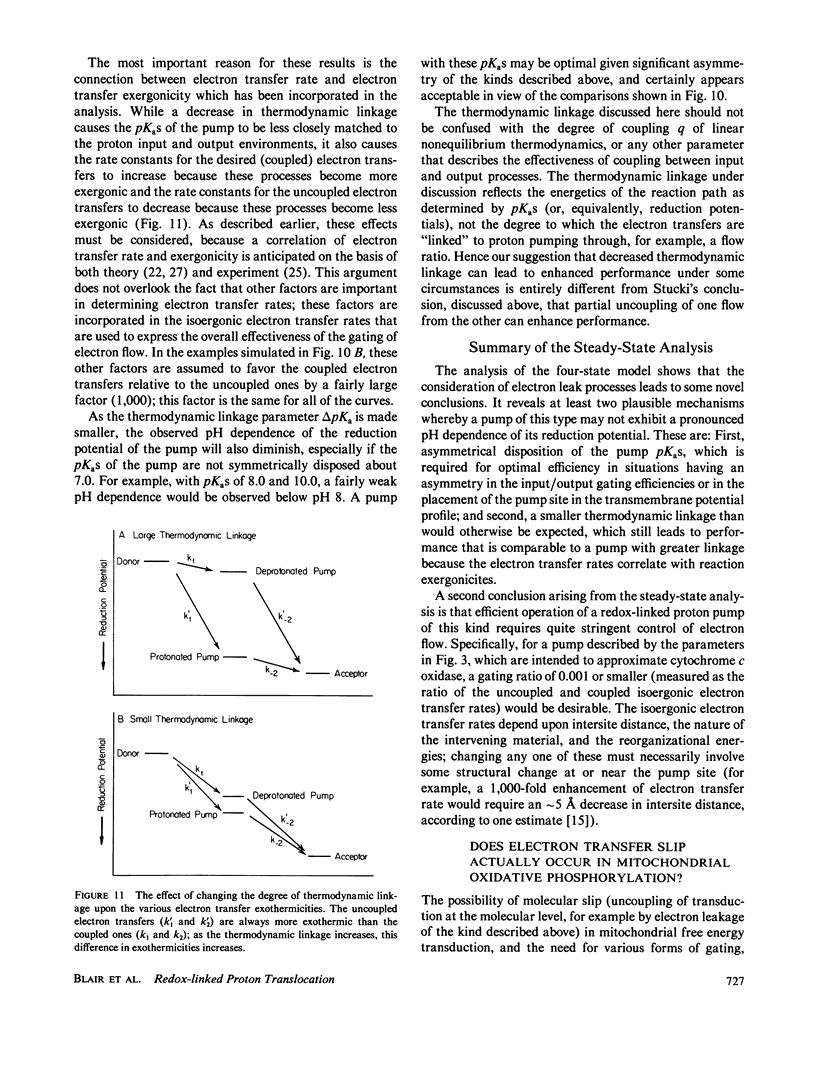
Abstract
In at least one component of the mitochondrial respiratory chain, cytochrome c oxidase, exothermic electron transfer reactions are used to drive vectorial proton transport against an electrochemical hydrogen ion gradient across the mitochondrial inner membrane. The role of the gating of electrons (the regulation of the rates of electron transfer into and out of the proton transport site) in this coupling between electron transfer and proton pumping has been explored. The approach involves the solution of the steady-state rate equations pertinent to proton pump models which include, to various degrees, the uncoupled (i.e., not linked to proton pumping) electron transfer processes which are likely to occur in any real electron transfer-driven proton pump. This analysis furnishes a quantitative framework for examining the effects of variations in proton binding site pKas and metal center reduction potentials, the relationship between energy conservation efficiency and turnover rate, the conditions for maximum power output or minimum heat production, and required efficiency of the gating of electrons. Some novel conclusions emerge from the analysis, including: An efficient electron transfer-driven proton pump need not exhibit a pH-dependent reduction potential; Very efficient gating of electrons is required for efficient electron transfer driven proton pumping, especially when a reasonable correlation of electron transfer rate and electron transfer exoergonicity is assumed; and A consideration of the importance and possible mechanisms of the gating of electrons suggests that efficient proton pumping by CuA in cytochrome oxidase could, in principle, take place with structural changes confined to the immediate vicinity of the copper ion, while proton pumping by Fea would probably require conformational coupling between the iron and more remote structures in the enzyme. The conclusions are discussed with reference to proton pumping by cytochrome c oxidase, and some possible implications for oxidative phosphorylation are noted.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Artzatbanov V. Y., Konstantinov A. A., Skulachev V. P. Involvement of intramitochondrial protons in redox reactions of cytochrome alpha. FEBS Lett. 1978 Mar 15;87(2):180–185. doi: 10.1016/0014-5793(78)80327-5. [DOI] [PubMed] [Google Scholar]
- Azzone G. F., Pozzan T., Massari S., Bragadin M. Proton electrochemical gradient and rate of controlled respiration in mitochondria. Biochim Biophys Acta. 1978 Feb 9;501(2):296–306. doi: 10.1016/0005-2728(78)90035-x. [DOI] [PubMed] [Google Scholar]
- Azzone G. F., Pozzan T., Massari S. Proton electrochemical gradient and phosphate potential in mitochondria. Biochim Biophys Acta. 1978 Feb 9;501(2):307–316. doi: 10.1016/0005-2728(78)90036-1. [DOI] [PubMed] [Google Scholar]
- Babcock G. T., Callahan P. M., Ondrias M. R., Salmeen I. Coordination geometries and vibrational properties of cytochromes alpha and alpha 3 in cytochrome oxidase from Soret excitation Raman spectroscopy. Biochemistry. 1981 Feb 17;20(4):959–966. doi: 10.1021/bi00507a049. [DOI] [PubMed] [Google Scholar]
- Babcock G. T., Callahan P. M. Redox-linked hydrogen bond strength changes in cytochrome a: implications for a cytochrome oxidase proton pump. Biochemistry. 1983 May 10;22(10):2314–2319. doi: 10.1021/bi00279a002. [DOI] [PubMed] [Google Scholar]
- Brudvig G. W., Blair D. F., Chan S. I. Electron spin relaxation of CuA and cytochrome a in cytochrome c oxidase. Comparison to heme, copper, and sulfur radical complexes. J Biol Chem. 1984 Sep 10;259(17):11001–11009. [PubMed] [Google Scholar]
- Brunori M., Sarti P., Colosimo A., Antonini G., Malatesta F., Jones M. G., Wilson M. T. Mechanism of control of cytochrome oxidase activity by the electrochemical-potential gradient. EMBO J. 1985 Sep;4(9):2365–2368. doi: 10.1002/j.1460-2075.1985.tb03940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. Respiratory enzymes in oxidative phosphorylation. I. Kinetics of oxygen utilization. J Biol Chem. 1955 Nov;217(1):383–393. [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- COPENHAVER J. H., Jr, LARDY H. A. Oxidative phosphorylations; pathways and yield in mitochondrial preparations. J Biol Chem. 1952 Mar;195(1):225–238. [PubMed] [Google Scholar]
- Casey R. P., Thelen M., Azzi A. Dicyclohexylcarbodiimide binds specifically and covalently to cytochrome c oxidase while inhibiting its H+-translocating activity. J Biol Chem. 1980 May 10;255(9):3994–4000. [PubMed] [Google Scholar]
- Devault D. Energy transduction in electron transport. Biochim Biophys Acta. 1971 Jan 12;226(1):193–199. doi: 10.1016/0005-2728(71)90192-7. [DOI] [PubMed] [Google Scholar]
- Duszyński J., Wojtczak L. The apparent non-linearity of the relationship between the rate of respiration and the protonmotive force of mitochondria can be explained by heterogeneity of mitochondrial preparations. FEBS Lett. 1985 Mar 25;182(2):243–248. doi: 10.1016/0014-5793(85)80307-0. [DOI] [PubMed] [Google Scholar]
- Dutton P. L. Redox potentiometry: determination of midpoint potentials of oxidation-reduction components of biological electron-transfer systems. Methods Enzymol. 1978;54:411–435. doi: 10.1016/s0076-6879(78)54026-3. [DOI] [PubMed] [Google Scholar]
- Ellis W. R., Jr, Wang H., Blair D. F., Gray H. B., Chan S. I. Spectroelectrochemical study of the cytochrome a site in carbon monoxide inhibited cytochrome c oxidase. Biochemistry. 1986 Jan 14;25(1):161–167. doi: 10.1021/bi00349a023. [DOI] [PubMed] [Google Scholar]
- Gelles J., Chan S. I. Chemical modification of the CuA center in cytochrome c oxidase by sodium p-(hydroxymercuri)benzoate. Biochemistry. 1985 Jul 16;24(15):3963–3972. doi: 10.1021/bi00336a025. [DOI] [PubMed] [Google Scholar]
- Hansen U. P., Gradmann D., Sanders D., Slayman C. L. Interpretation of current-voltage relationships for "active" ion transport systems: I. Steady-state reaction-kinetic analysis of class-I mechanisms. J Membr Biol. 1981;63(3):165–190. doi: 10.1007/BF01870979. [DOI] [PubMed] [Google Scholar]
- Hill T. L. Some general principles in free energy transduction. Proc Natl Acad Sci U S A. 1983 May;80(10):2922–2925. doi: 10.1073/pnas.80.10.2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfield J. J. Electron transfer between biological molecules by thermally activated tunneling. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3640–3644. doi: 10.1073/pnas.71.9.3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppe J., Sebald W. The proton conducting F0-part of bacterial ATP synthases. Biochim Biophys Acta. 1984 Apr 9;768(1):1–27. doi: 10.1016/0304-4173(84)90005-3. [DOI] [PubMed] [Google Scholar]
- Hunter D. R., Capaldi R. A. Respiratory control in cytochrome oxidase. Biochem Biophys Res Commun. 1974 Feb 4;56(3):623–628. doi: 10.1016/0006-291x(74)90650-0. [DOI] [PubMed] [Google Scholar]
- Kagawa Y. Reconstitution of the energy transformer, gate and channel subunit reassembly, crystalline ATPase and ATP synthesis. Biochim Biophys Acta. 1978 Sep 21;505(1):45–93. doi: 10.1016/0304-4173(78)90008-3. [DOI] [PubMed] [Google Scholar]
- Kell D. B., John P., Ferguson S. J. The protonmotive force in phosphorylating membrane vesicles from Paracoccus denitrificans. Magnitude, sites of generation and comparison with the phosphorylation potential. Biochem J. 1978 Jul 15;174(1):257–266. doi: 10.1042/bj1740257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima N., Palmer G. Further characterization of the potentiometric behavior of cytochrome oxidase. Cytochrome alpha stays low spin during oxidation and reduction. J Biol Chem. 1983 Dec 25;258(24):14908–14913. [PubMed] [Google Scholar]
- Läuger P. A channel mechanism for electrogenic ion pumps. Biochim Biophys Acta. 1979 Mar 23;552(1):143–161. doi: 10.1016/0005-2736(79)90253-0. [DOI] [PubMed] [Google Scholar]
- Läuger P., Stark G. Kinetics of carrier-mediated ion transport across lipid bilayer membranes. Biochim Biophys Acta. 1970 Sep 15;211(3):458–466. doi: 10.1016/0005-2736(70)90251-8. [DOI] [PubMed] [Google Scholar]
- MITCHELL P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961 Jul 8;191:144–148. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- Makinen M. W., Schichman S. A., Hill S. C., Gray H. B. Heme-heme orientation and electron transfer kinetic behavior of multisite oxidation-reduction enzymes. Science. 1983 Nov 25;222(4626):929–931. doi: 10.1126/science.6415814. [DOI] [PubMed] [Google Scholar]
- Malmström B. G. Hypothesis. Cytochrome c oxidase as a proton pump. A transition-state mechanism. Biochim Biophys Acta. 1985 Apr 8;811(1):1–12. [PubMed] [Google Scholar]
- Maloney P. C. Energy coupling to ATP synthesis by the proton-translocating ATPase. J Membr Biol. 1982;67(1):1–12. doi: 10.1007/BF01868643. [DOI] [PubMed] [Google Scholar]
- Margalit R., Kostić N. M., Che C. M., Blair D. F., Chiang H. J., Pecht I., Shelton J. B., Shelton J. R., Schroeder W. A., Gray H. B. Preparation and characterization of pentaammineruthenium-(histidine-83)azurin: thermodynamics of intramolecular electron transfer from ruthenium to copper. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6554–6558. doi: 10.1073/pnas.81.20.6554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C. T., Scholes C. P., Chan S. I. The identification of histidine ligands to cytochrome a in cytochrome c oxidase. J Biol Chem. 1985 Mar 10;260(5):2857–2861. [PubMed] [Google Scholar]
- Meyer T. E., Przysiecki C. T., Watkins J. A., Bhattacharyya A., Simondsen R. P., Cusanovich M. A., Tollin G. Correlation between rate constant for reduction and redox potential as a basis for systematic investigation of reaction mechanisms of electron transfer proteins. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6740–6744. doi: 10.1073/pnas.80.22.6740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Estimation of membrane potential and pH difference across the cristae membrane of rat liver mitochondria. Eur J Biochem. 1969 Feb;7(4):471–484. doi: 10.1111/j.1432-1033.1969.tb19633.x. [DOI] [PubMed] [Google Scholar]
- Nelson N. Proton channels in chloroplast membranes. Ann N Y Acad Sci. 1980;358:25–35. doi: 10.1111/j.1749-6632.1980.tb15383.x. [DOI] [PubMed] [Google Scholar]
- Nicholls D. G. Brown adipose tissue mitochondria. Biochim Biophys Acta. 1979 Jul 3;549(1):1–29. doi: 10.1016/0304-4173(79)90016-8. [DOI] [PubMed] [Google Scholar]
- Nicholls D. G. The influence of respiration and ATP hydrolysis on the proton-electrochemical gradient across the inner membrane of rat-liver mitochondria as determined by ion distribution. Eur J Biochem. 1974 Dec 16;50(1):305–315. doi: 10.1111/j.1432-1033.1974.tb03899.x. [DOI] [PubMed] [Google Scholar]
- Okamoto H., Sone N., Hirata H., Yoshida M., Kagawa Y. Purified proton conductor in proton translocating adenosine triphosphatase of a thermophilic bacterium. J Biol Chem. 1977 Sep 10;252(17):6125–6131. [PubMed] [Google Scholar]
- Papa S. Proton translocation reactions in the respiratory chains. Biochim Biophys Acta. 1976 Apr 30;456(1):39–84. doi: 10.1016/0304-4173(76)90008-2. [DOI] [PubMed] [Google Scholar]
- Pietrobon D., Azzone G. F., Walz D. Effect of funiculosin and antimycin A on the redox-driven H+-pumps in mitochondria: on the nature of "leaks'. Eur J Biochem. 1981 Jul;117(2):389–394. doi: 10.1111/j.1432-1033.1981.tb06350.x. [DOI] [PubMed] [Google Scholar]
- Pietrobon D., Caplan S. R. Flow-force relationships for a six-state proton pump model: intrinsic uncoupling, kinetic equivalence of input and output forces, and domain of approximate linearity. Biochemistry. 1985 Oct 8;24(21):5764–5776. doi: 10.1021/bi00342a012. [DOI] [PubMed] [Google Scholar]
- Pietrobon D., Zoratti M., Azzone G. F. Molecular slipping in redox and ATPase H+ pumps. Biochim Biophys Acta. 1983 May 27;723(2):317–321. doi: 10.1016/0005-2728(83)90131-7. [DOI] [PubMed] [Google Scholar]
- Pietrobon D., Zoratti M., Azzone G. F., Stucki J. W., Walz D. Non-equilibrium thermodynamic assessment of redox-driven H+ pumps in mitochondria. Eur J Biochem. 1982 Oct;127(3):483–494. doi: 10.1111/j.1432-1033.1982.tb06897.x. [DOI] [PubMed] [Google Scholar]
- Poulos T. L., Kraut J. A hypothetical model of the cytochrome c peroxidase . cytochrome c electron transfer complex. J Biol Chem. 1980 Nov 10;255(21):10322–10330. [PubMed] [Google Scholar]
- Rottenberg H. Non-equilibrium thermodynamics of energy conversion in bioenergetics. Biochim Biophys Acta. 1979 Dec 13;549(3-4):225–253. doi: 10.1016/0304-4173(79)90001-6. [DOI] [PubMed] [Google Scholar]
- Scheiner S. Quantum chemical studies of proton transport through biomembranes. Ann N Y Acad Sci. 1981;367:493–509. doi: 10.1111/j.1749-6632.1981.tb50586.x. [DOI] [PubMed] [Google Scholar]
- Slater E. C., Rosing J., Mol A. The phosphorylation potential generated by respiring mitochondria. Biochim Biophys Acta. 1973 Apr 5;292(3):534–553. doi: 10.1016/0005-2728(73)90003-0. [DOI] [PubMed] [Google Scholar]
- Sorgato M. C., Branca D., Ferguson S. J. The rate of ATP synthesis by submitochondrial particles can be independent of the magnitude of the protonmotive force. Biochem J. 1980 Jun 15;188(3):945–948. doi: 10.1042/bj1880945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorgato M. C., Ferguson S. J., Kell D. B., John P. The protonmotive force in bovine heart submitochondrial particles. Magnitude, sites of generation and comparison with the phosphorylation potential. Biochem J. 1978 Jul 15;174(1):237–256. doi: 10.1042/bj1740237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorgato M. C., Ferguson S. J. Variable proton conductance of submitochondrial particles. Biochemistry. 1979 Dec 11;18(25):5737–5742. doi: 10.1021/bi00592a034. [DOI] [PubMed] [Google Scholar]
- Stucki J. W. The optimal efficiency and the economic degrees of coupling of oxidative phosphorylation. Eur J Biochem. 1980 Aug;109(1):269–283. doi: 10.1111/j.1432-1033.1980.tb04792.x. [DOI] [PubMed] [Google Scholar]
- Thelen M., O'Shea P. S., Azzi A. New insights on the cytochrome c oxidase proton pump. Biochem J. 1985 Apr 1;227(1):163–167. doi: 10.1042/bj2270163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vik S. B., Capaldi R. A. Conditions for optimal electron transfer activity of cytochrome c oxidase isolated from beef heart mitochondria. Biochem Biophys Res Commun. 1980 May 14;94(1):348–354. doi: 10.1016/s0006-291x(80)80227-0. [DOI] [PubMed] [Google Scholar]
- Wang H., Blair D. F., Ellis W. R., Jr, Gray H. B., Chan S. I. Temperature dependence of the reduction potential of CuA in carbon monoxide inhibited cytochrome c oxidase. Biochemistry. 1986 Jan 14;25(1):167–171. doi: 10.1021/bi00349a024. [DOI] [PubMed] [Google Scholar]
- Wikstrom M. K. Proton pump coupled to cytochrome c oxidase in mitochondria. Nature. 1977 Mar 17;266(5599):271–273. doi: 10.1038/266271a0. [DOI] [PubMed] [Google Scholar]
- Wikström M., Krab K. Proton-pumping cytochrome c oxidase. Biochim Biophys Acta. 1979 Aug 17;549(2):177–122. doi: 10.1016/0304-4173(79)90014-4. [DOI] [PubMed] [Google Scholar]
- Wikström M., Krab K., Saraste M. Proton-translocating cytochrome complexes. Annu Rev Biochem. 1981;50:623–655. doi: 10.1146/annurev.bi.50.070181.003203. [DOI] [PubMed] [Google Scholar]


