Abstract
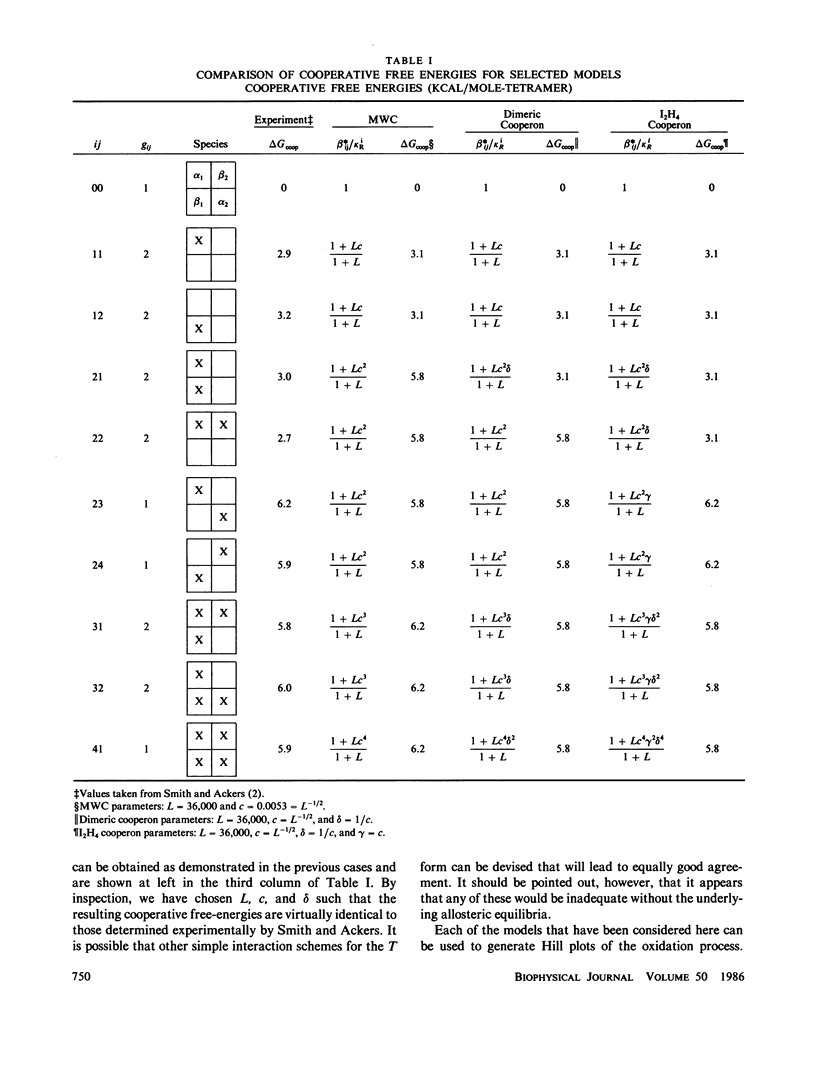
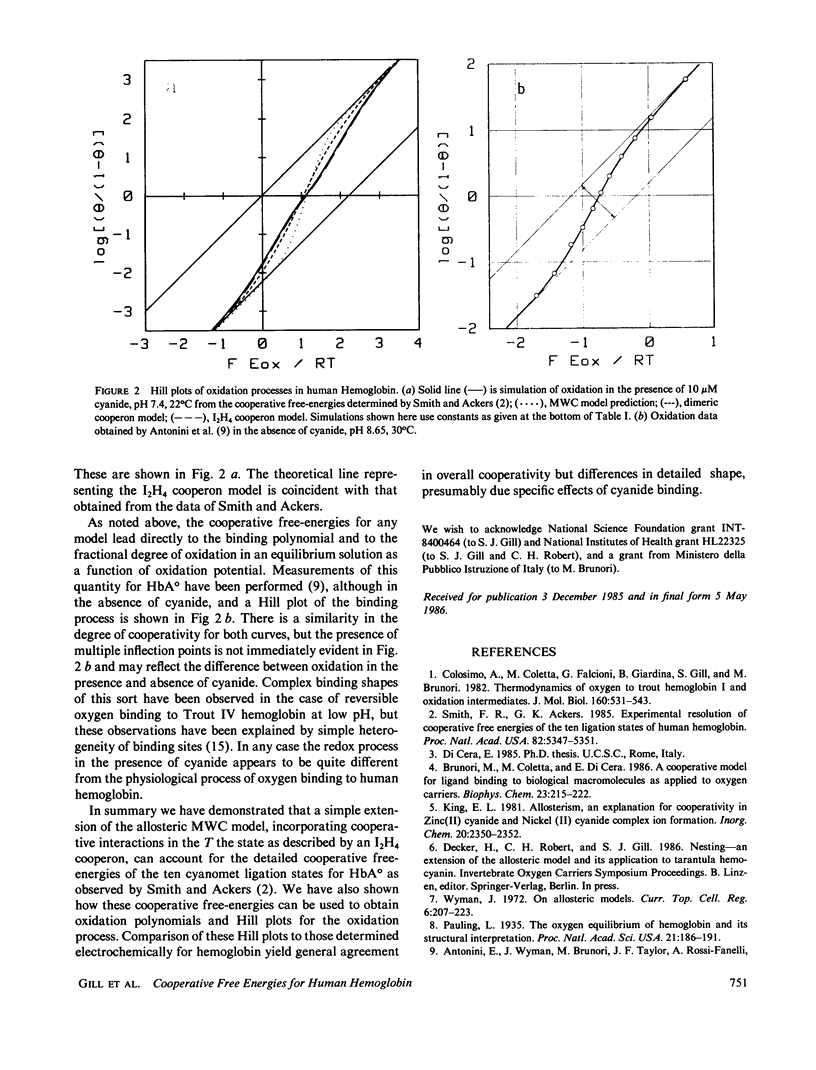
A model is developed for ligand binding to human hemoglobin that describes the detailed cooperative free-energies for each of the ten different ligated (cyanomet) species as observed by Smith and Ackers (Smith, F.R., and G.K. Ackers. 1985. Proc. Natl. Acad. Sci. USA.82:5347-5351). The approach taken here is an application of the general principle of hierarchical levels of allosteric control, or nesting, as suggested by Wyman (Wyman, J. 1972. Curr. Top. Cell. Reg. 6:207-223). The model is an extension of the simple two-state MWC model (Monod, J., J. Wyman, and J.P. Changeux. 1965. J. Mol. Biol. 12:88-118) using the idea of cooperative binding within the T (deoxy) form of the macromolecule, and has recently been described as a "cooperon" model (Di Cera, E. 1985. Ph.D. thesis). The T-state cooperative binding is described using simple interaction rules first devised by Pauling (Pauling, L. 1935. Proc. Natl. Acad. Sci. USA. 21:186-191). In this application three parameters suffice to describe the cooperative free-energies of the 10 ligated species of cyanomet hemoglobin. The redox process in the presence of cyanide, represented as a Hill plot, is simulated from Smith and Ackers' cooperative free-energies and is compared with available electrochemical binding measurements.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANTONINI E., WYMAN J., BRUNORI M., TAYLOR J. F., ROSSI-FANELLI A., CAPUTO A. STUDIES ON THE OXIDATION-REDUCTION POTENTIALS OF HEME PROTEINS. I. HUMAN HEMOGLOBIN. J Biol Chem. 1964 Mar;239:907–912. [PubMed] [Google Scholar]
- Ackers G. K., Halvorson H. R. The linkage between oxygenation and subunit dissociation in human hemoglobin. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4312–4316. doi: 10.1073/pnas.71.11.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunori M., Coletta M., Di Cera E. A cooperative model for ligand binding to biological macromolecules as applied to oxygen carriers. Biophys Chem. 1986 Mar;23(3-4):215–222. doi: 10.1016/0301-4622(86)85006-2. [DOI] [PubMed] [Google Scholar]
- Brunori M., Coletta M., Giardina B., Wyman J. A macromolecular transducer as illustrated by trout hemoglobin IV. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4310–4312. doi: 10.1073/pnas.75.9.4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colosimo A., Coletta M., Falcioni G., Giardina B., Gill S. J., Brunori M. Thermodynamics of oxygen binding to trout haemoglobin I and its oxidation intermediates. J Mol Biol. 1982 Sep 25;160(3):531–543. doi: 10.1016/0022-2836(82)90312-6. [DOI] [PubMed] [Google Scholar]
- Cornish-Bowden A., Koshland D. E., Jr The influence of binding domains on the nature of subunit interactions in oligomeric proteins. Application to unusual kinetic and binding patterns. J Biol Chem. 1970 Dec 10;245(23):6241–6250. [PubMed] [Google Scholar]
- Koshland D. E., Jr, Némethy G., Filmer D. Comparison of experimental binding data and theoretical models in proteins containing subunits. Biochemistry. 1966 Jan;5(1):365–385. doi: 10.1021/bi00865a047. [DOI] [PubMed] [Google Scholar]
- Pauling L. The Oxygen Equilibrium of Hemoglobin and Its Structural Interpretation. Proc Natl Acad Sci U S A. 1935 Apr;21(4):186–191. doi: 10.1073/pnas.21.4.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith F. R., Ackers G. K. Experimental resolution of cooperative free energies for the ten ligation states of human hemoglobin. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5347–5351. doi: 10.1073/pnas.82.16.5347. [DOI] [PMC free article] [PubMed] [Google Scholar]


