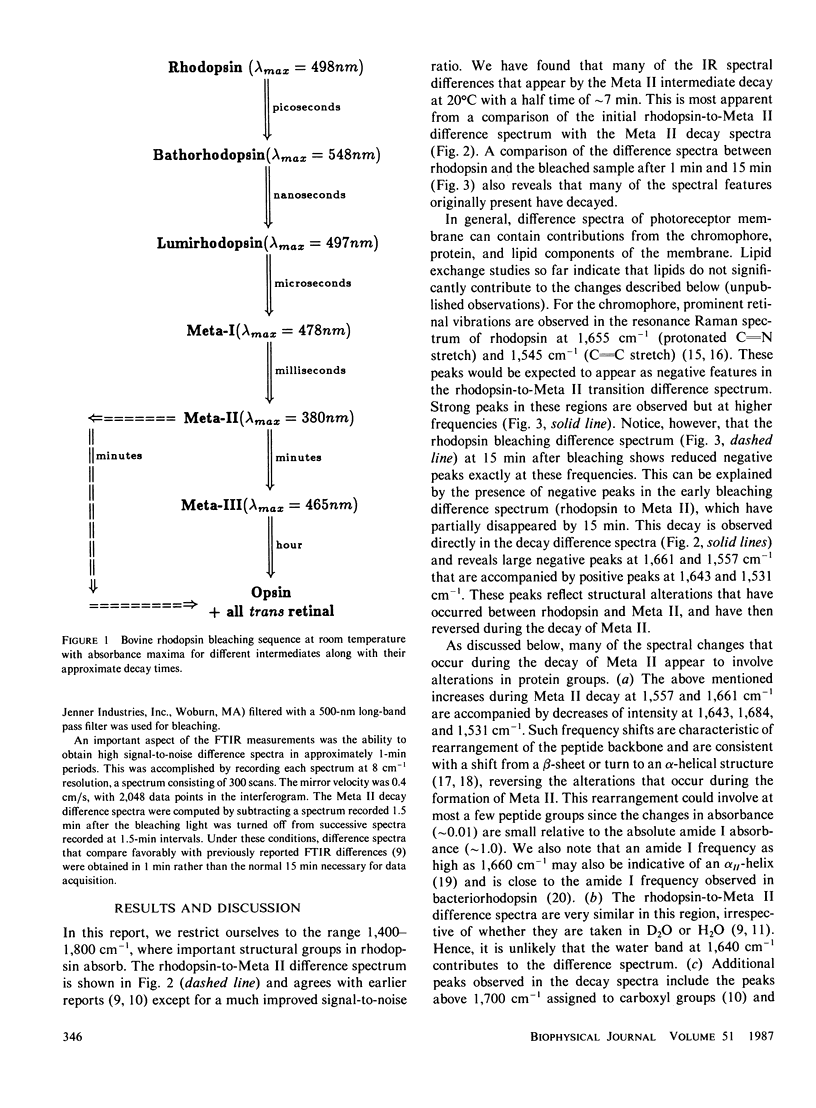
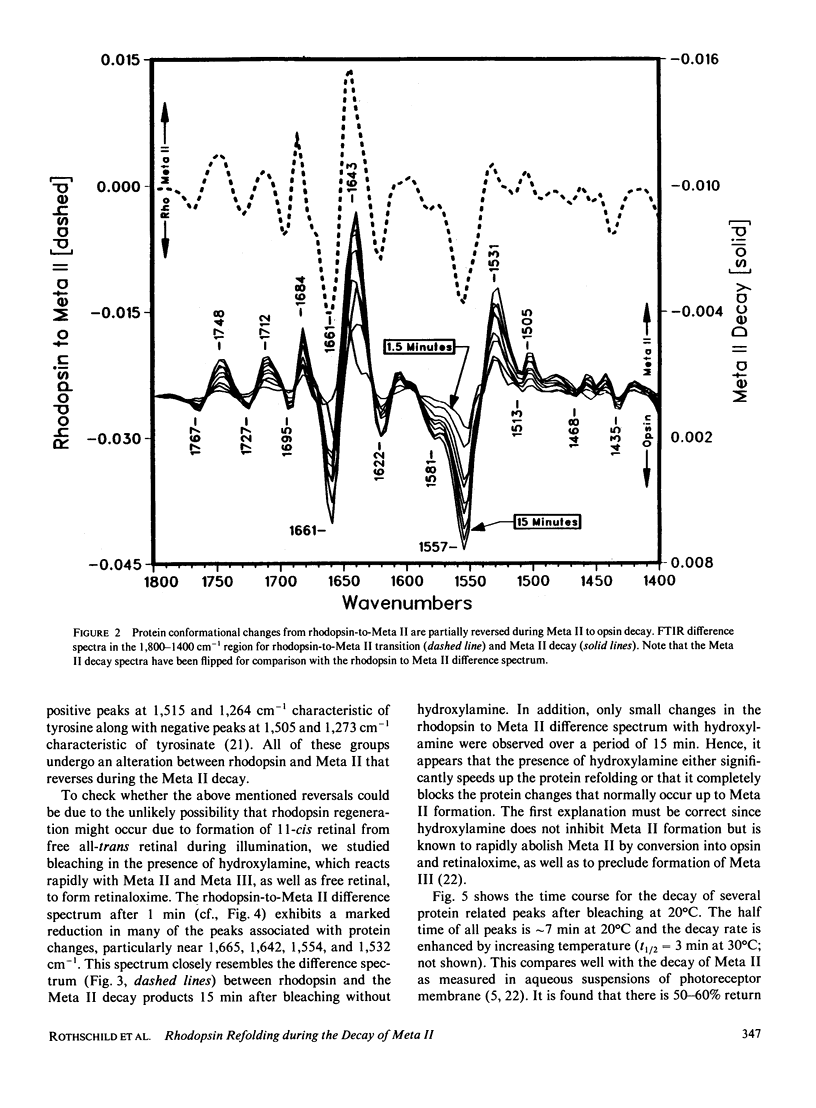
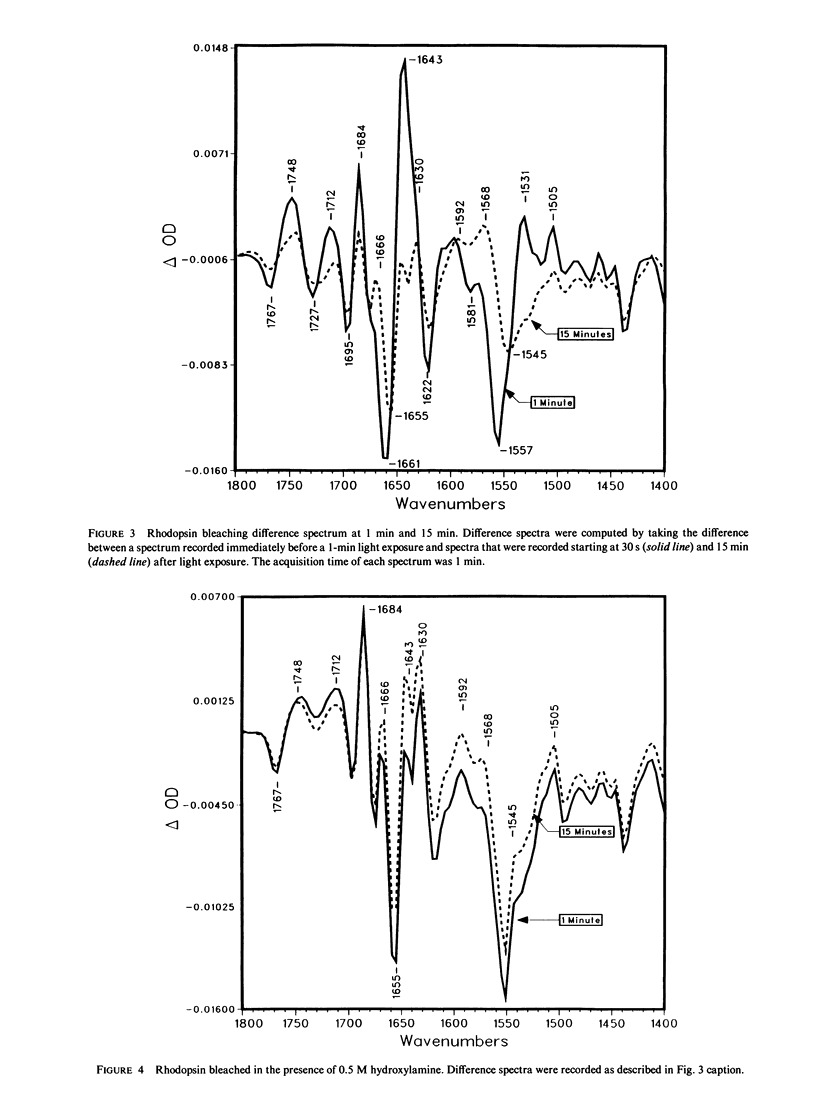
Abstract
Fourier transform infrared difference spectroscopy (FTIR) reveals that the Meta II intermediate of the rhodopsin bleaching cascade is structurally distorted relative to rhodopsin. In addition to previously detected alterations in the state of carboxyl groups, a small part of the protein back-bone undergoes a conversion from alpha-helical to beta-type structure. All of these changes partially reverse during Meta II decay. This evidence together with FTIR studies of earlier photointermediates indicates that of the known photointermediates the protein structure of Meta II is the most distorted. It is concluded that light causes rhodopsin to convert into a conformationally distorted form (Meta II), which subsequently refolds into a more rhodopsin-like conformation (opsin).
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagley K. A., Balogh-Nair V., Croteau A. A., Dollinger G., Ebrey T. G., Eisenstein L., Hong M. K., Nakanishi K., Vittitow J. Fourier-transform infrared difference spectroscopy of rhodopsin and its photoproducts at low temperature. Biochemistry. 1985 Oct 22;24(22):6055–6071. doi: 10.1021/bi00343a006. [DOI] [PubMed] [Google Scholar]
- Clark N. A., Rothschild K. J., Luippold D. A., Simon B. A. Surface-induced lamellar orientation of multilayer membrane arrays. Theoretical analysis and a new method with application to purple membrane fragments. Biophys J. 1980 Jul;31(1):65–96. doi: 10.1016/S0006-3495(80)85041-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper A. Energy uptake in the first step of visual excitation. Nature. 1979 Nov 29;282(5738):531–533. doi: 10.1038/282531a0. [DOI] [PubMed] [Google Scholar]
- Doukas A. G., Aton B., Callender R. H., Ebrey T. G. Resonance Raman studies of bovine metarhodopsin I and metarhodopsin II. Biochemistry. 1978 Jun 13;17(12):2430–2435. doi: 10.1021/bi00605a028. [DOI] [PubMed] [Google Scholar]
- Krimm S., Dwivedi A. M. Infrared spectrum of the purple membrane: clue to a proton conduction mechanism? Science. 1982 Apr 23;216(4544):407–408. doi: 10.1126/science.6280277. [DOI] [PubMed] [Google Scholar]
- Krimm S. Vibrational analysis of conformation in peptides, polypeptides, and proteins. Biopolymers. 1983 Jan;22(1):217–225. doi: 10.1002/bip.360220130. [DOI] [PubMed] [Google Scholar]
- MATTHEWS R. G., HUBBARD R., BROWN P. K., WALD G. TAUTOMERIC FORMS OF METARHODOPSIN. J Gen Physiol. 1963 Nov;47:215–240. doi: 10.1085/jgp.47.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathies R., Oseroff A. R., Stryer L. Rapid-flow resonance Raman spectroscopy of photolabile molecules: rhodopsin and isorhodopsin. Proc Natl Acad Sci U S A. 1976 Jan;73(1):1–5. doi: 10.1073/pnas.73.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostroy S. E. Rhodopsin and the visual process. Biochim Biophys Acta. 1977 Jun 21;463(1):91–125. doi: 10.1016/0304-4173(77)90004-0. [DOI] [PubMed] [Google Scholar]
- Rothschild K. J., Cantore W. A., Marrero H. Fourier transform infrared difference spectra of intermediates in rhodopsin bleaching. Science. 1983 Mar 18;219(4590):1333–1335. doi: 10.1126/science.6828860. [DOI] [PubMed] [Google Scholar]
- Rothschild K. J., Clark N. A. Anomalous amide I infrared absorption of purple membrane. Science. 1979 Apr 20;204(4390):311–312. doi: 10.1126/science.432645. [DOI] [PubMed] [Google Scholar]
- Rothschild K. J., Roepe P., Ahl P. L., Earnest T. N., Bogomolni R. A., Das Gupta S. K., Mulliken C. M., Herzfeld J. Evidence for a tyrosine protonation change during the primary phototransition of bacteriorhodopsin at low temperature. Proc Natl Acad Sci U S A. 1986 Jan;83(2):347–351. doi: 10.1073/pnas.83.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothschild K. J., Rosen K. M., Clark N. A. Incorporation of photoreceptor membrane into a multilamellar film. Biophys J. 1980 Jul;31(1):45–52. doi: 10.1016/S0006-3495(80)85039-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebert F., Mäntele W., Gerwert K. Fourier-transform infrared spectroscopy applied to rhodopsin. The problem of the protonation state of the retinylidene Schiff base re-investigated. Eur J Biochem. 1983 Oct 17;136(1):119–127. doi: 10.1111/j.1432-1033.1983.tb07714.x. [DOI] [PubMed] [Google Scholar]
- Stryer L. Molecular design of an amplification cascade in vision. Biopolymers. 1985 Jan;24(1):29–47. doi: 10.1002/bip.360240105. [DOI] [PubMed] [Google Scholar]
- Wald G. The molecular basis of visual excitation. Nature. 1968 Aug 24;219(5156):800–807. doi: 10.1038/219800a0. [DOI] [PubMed] [Google Scholar]
- de Grip W. J., Gillespie J., Rothschild K. J. Carboxyl group involvement in the meta I and meta II stages in rhodopsin bleaching. A Fourier transform infrared spectroscopic study. Biochim Biophys Acta. 1985 Aug 28;809(1):97–106. doi: 10.1016/0005-2728(85)90172-0. [DOI] [PubMed] [Google Scholar]
- van Breugel P. J., Bovee-Geurts P. H., Bonting S. L., Daemen F. J. Biochemical aspects of the visual process. XL. Spectral and chemical analysis of metarhodopsin III in photoreceptor membrane suspensions. Biochim Biophys Acta. 1979 Oct 19;557(1):188–198. doi: 10.1016/0005-2736(79)90101-9. [DOI] [PubMed] [Google Scholar]


