Abstract
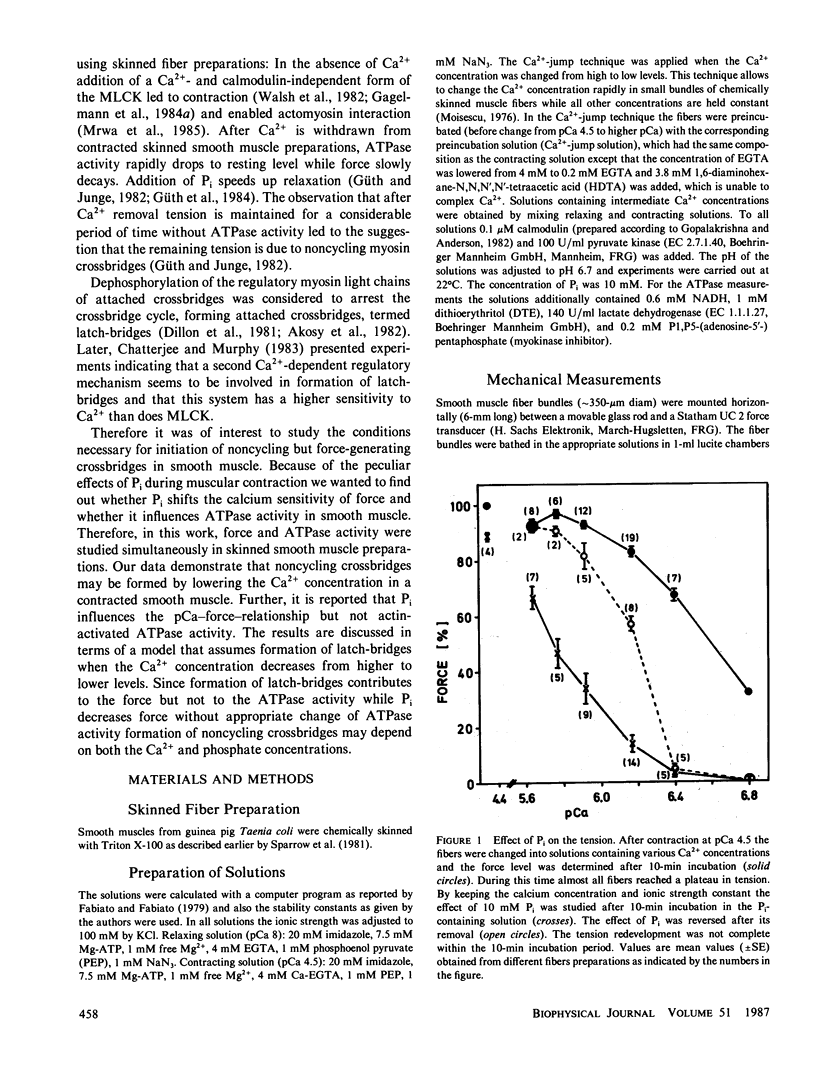
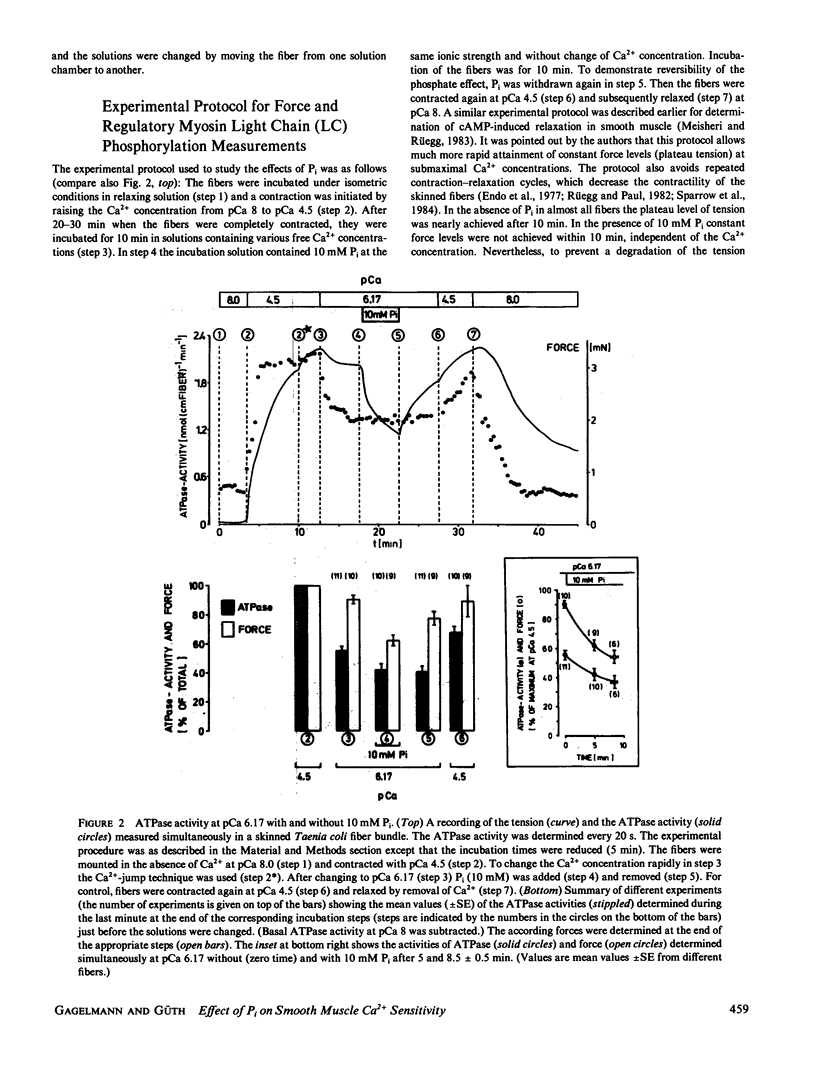
Inorganic phosphate (Pi) decreases maximal tension in contracted skeletal and heart muscle fibers. We investigated the effects of 10 mM Pi on the force-calcium relationship in Triton X-100-skinned Taenia coli smooth muscle fibers. Isometric force measurements show that the calcium sensitivity of the force depends on the phosphate concentration. Furthermore 10 mM Pi relaxes the fibers more at intermediate than at high calcium ion concentrations: At pCa 4.5 tension decreases in the presence of 10 mM Pi by approximately 12% but it decreases 70% at pCa 6.17. Removal of phosphate partially reverses the relaxation. Simultaneous determination of actomyosin ATPase activity and force (Güth, K., and J. Junge, 1982, Nature (Lond.), 300:775-776) shows that the ATPase activity does not correlate with the changes in force. In the presence of Pi, tension decreases more than the ATPase activity. The level of phosphorylation of the 20,000-D regulatory myosin light chain is not changed in the presence or absence of 10 mM Pi. The results are discussed in terms of slowly or noncycling myosin crossbridges formed at lower calcium concentrations, which contribute to the force development but not to the ATPase activity. These crossbridges are considered to be dissociated in the presence of phosphate.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aksoy M. O., Murphy R. A., Kamm K. E. Role of Ca2+ and myosin light chain phosphorylation in regulation of smooth muscle. Am J Physiol. 1982 Jan;242(1):C109–C116. doi: 10.1152/ajpcell.1982.242.1.C109. [DOI] [PubMed] [Google Scholar]
- Chatterjee M., Murphy R. A. Calcium-dependent stress maintenance without myosin phosphorylation in skinned smooth muscle. Science. 1983 Jul 29;221(4609):464–466. doi: 10.1126/science.6867722. [DOI] [PubMed] [Google Scholar]
- Dillon P. F., Aksoy M. O., Driska S. P., Murphy R. A. Myosin phosphorylation and the cross-bridge cycle in arterial smooth muscle. Science. 1981 Jan 30;211(4481):495–497. doi: 10.1126/science.6893872. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Gagelmann M., Güth K. Force generated by non-cycling crossbridges at low ionic strength in skinned smooth muscle from Taenia coli. Pflugers Arch. 1985 Feb;403(2):210–214. doi: 10.1007/BF00584102. [DOI] [PubMed] [Google Scholar]
- Gagelmann M., Rüegg J. C., Di Salvo J. Phosphorylation of the myosin light chains and satellite proteins in detergent-skinned arterial smooth muscle. Biochem Biophys Res Commun. 1984 May 16;120(3):933–938. doi: 10.1016/s0006-291x(84)80196-5. [DOI] [PubMed] [Google Scholar]
- Gopalakrishna R., Anderson W. B. Ca2+-induced hydrophobic site on calmodulin: application for purification of calmodulin by phenyl-Sepharose affinity chromatography. Biochem Biophys Res Commun. 1982 Jan 29;104(2):830–836. doi: 10.1016/0006-291x(82)90712-4. [DOI] [PubMed] [Google Scholar]
- Güth K., Gagelmann M., Rüegg J. C. Skinned smooth muscle: time course of force and ATPase activity during contraction cycle. Experientia. 1984 Feb 15;40(2):174–176. doi: 10.1007/BF01963585. [DOI] [PubMed] [Google Scholar]
- Güth K., Junge J. Low Ca2+ impedes cross-bridge detachment in chemically skinned Taenia coli. Nature. 1982 Dec 23;300(5894):775–776. doi: 10.1038/300775a0. [DOI] [PubMed] [Google Scholar]
- Güth K., Wojciechowski R. Perfusion cuvette for the simultaneous measurement of mechanical, optical and energetic parameters of skinned muscle fibres. Pflugers Arch. 1986 Nov;407(5):552–557. doi: 10.1007/BF00657515. [DOI] [PubMed] [Google Scholar]
- Jacobus W. E., Taylor G. J., 4th, Hollis D. P., Nunnally R. L. Phosphorus nuclear magnetic resonance of perfused working rat hearts. Nature. 1977 Feb 24;265(5596):756–758. doi: 10.1038/265756a0. [DOI] [PubMed] [Google Scholar]
- Krisanda J. M., Paul R. J. Phosphagen and metabolite content during contraction in porcine carotid artery. Am J Physiol. 1983 May;244(5):C385–C390. doi: 10.1152/ajpcell.1983.244.5.C385. [DOI] [PubMed] [Google Scholar]
- Kübler W., Katz A. M. Mechanism of early "pump" failure of the ischemic heart: possible role of adenosine triphosphate depletion and inorganic phosphate accumulation. Am J Cardiol. 1977 Sep;40(3):467–471. doi: 10.1016/0002-9149(77)90174-6. [DOI] [PubMed] [Google Scholar]
- Meisheri K. D., Ruegg J. C. Dependence of cyclic-AMP induced relaxation on Ca2+ and calmodulin in skinned smooth muscle of guinea pig Taenia coli. Pflugers Arch. 1983 Dec;399(4):315–320. doi: 10.1007/BF00652759. [DOI] [PubMed] [Google Scholar]
- Moisescu D. G. Kinetics of reaction in calcium-activated skinned muscle fibres. Nature. 1976 Aug 12;262(5569):610–613. doi: 10.1038/262610a0. [DOI] [PubMed] [Google Scholar]
- Mrwa U., Güth K., Rüegg J. C., Paul R. J., Boström S., Barsotti R., Hartshorne D. Mechanical and biochemical characterization of the contraction elicited by a calcium-independent myosin light chain kinase in chemically skinned smooth muscle. Experientia. 1985 Aug 15;41(8):1002–1006. doi: 10.1007/BF01952121. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Rüegg J. C., Paul R. J. Vascular smooth muscle. Calmodulin and cyclic AMP-dependent protein kinase after calcium sensitivity in porcine carotid skinned fibers. Circ Res. 1982 Mar;50(3):394–399. doi: 10.1161/01.res.50.3.394. [DOI] [PubMed] [Google Scholar]
- Schneider M., Sparrow M., Rüegg J. C. Inorganic phosphate promotes relaxation of chemically skinned smooth muscle of guinea-pig Taenia coli. Experientia. 1981;37(9):980–982. doi: 10.1007/BF01971791. [DOI] [PubMed] [Google Scholar]
- Sparrow M. P., Mrwa U., Hofmann F., Rüegg J. C. Calmodulin is essential for smooth muscle contraction. FEBS Lett. 1981 Mar 23;125(2):141–145. doi: 10.1016/0014-5793(81)80704-1. [DOI] [PubMed] [Google Scholar]
- Sparrow M. P., Pfitzer G., Gagelmann M., Rüegg J. C. Effect of calmodulin, Ca2+, and cAMP protein kinase on skinned tracheal smooth muscle. Am J Physiol. 1984 Mar;246(3 Pt 1):C308–C314. doi: 10.1152/ajpcell.1984.246.3.C308. [DOI] [PubMed] [Google Scholar]
- Walsh M. P., Bridenbaugh R., Hartshorne D. J., Kerrick W. G. Phosphorylation-dependent activated tension in skinned gizzard muscle fibers in the absence of Ca2+. J Biol Chem. 1982 Jun 10;257(11):5987–5990. [PubMed] [Google Scholar]
- Wilkie D. R., Dawson M. J., Edwards R. H., Gordon R. E., Shaw D. 31P NMR studies of resting muscle in normal human subjects. Adv Exp Med Biol. 1984;170:333–347. doi: 10.1007/978-1-4684-4703-3_28. [DOI] [PubMed] [Google Scholar]


