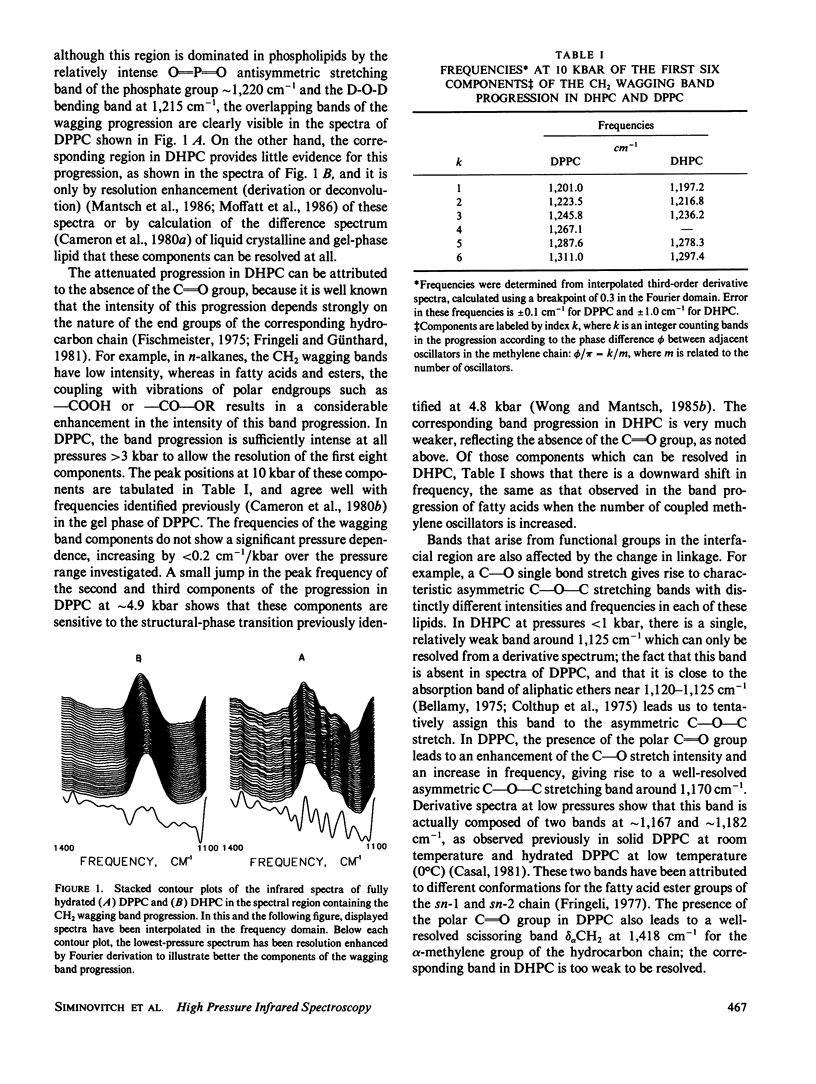
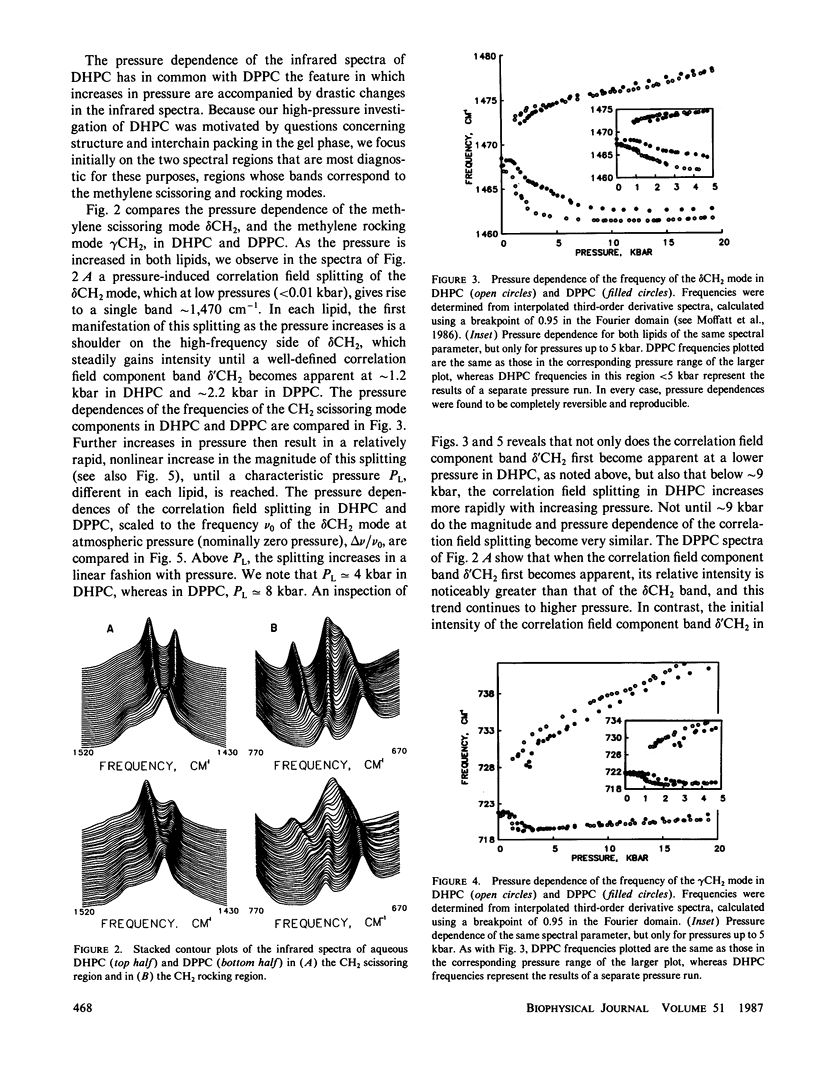
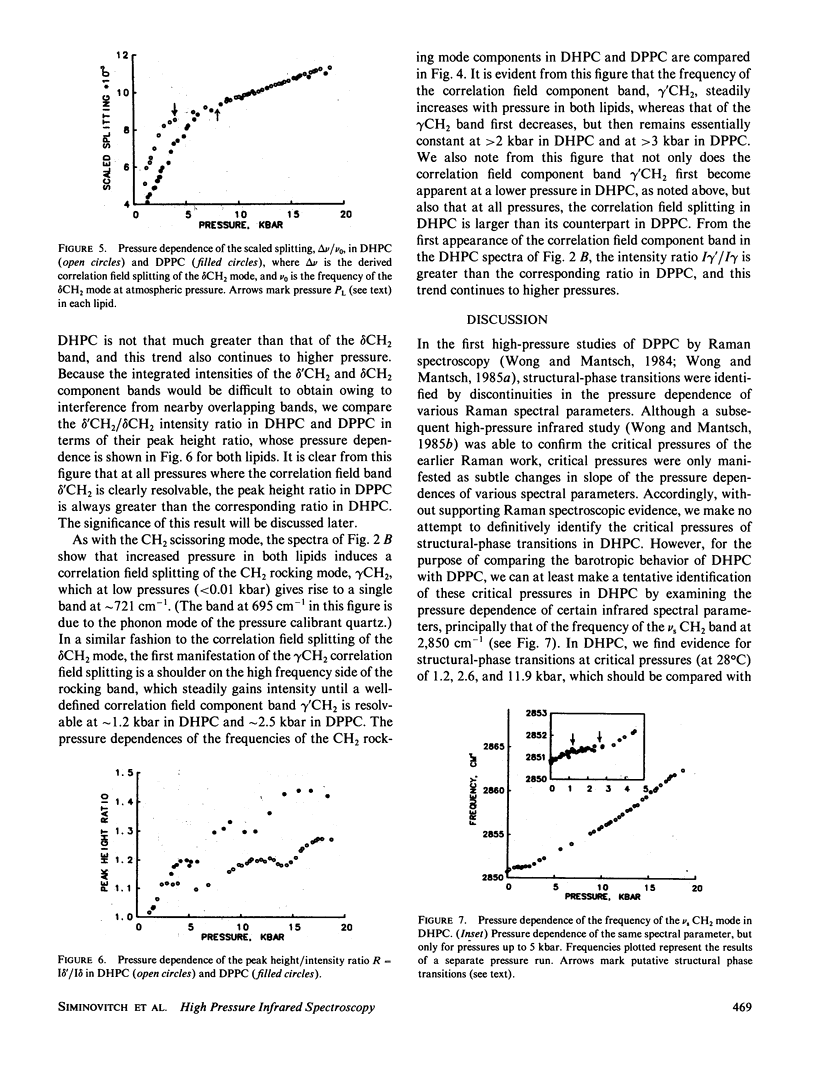
Abstract
Infrared spectra of aqueous dispersions of 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), and its ether-linked analogue, 1,2-dihexadecyl-sn-glycero-3-phosphocholine (DHPC), were measured in a diamond anvil cell at 28 degrees C as a function of pressure up to 20 kbar. Although these two lipids differ only in the linkages to the saturated hydrocarbon chains, significant differences were observed in their barotropic behavior. Most notable were the magnitudes of the pressure-induced correlation field splittings of the methylene scissoring and rocking modes, and the relative intensities of the corresponding component bands. In the case of the scissoring mode, not only can the correlation field component band be resolved at a lower pressure in DHPC (1.2 kbar, as compared with 2.2 kbar in DPPC), but the initial magnitude of the correlation field splitting in DHPC, particularly less than 9 kbar, is significantly greater than that observed in DPPC. These differences are attributed to the presence of an interdigitated lamellar gel phase in DHPC. At all pressures where the correlation field component band delta'CH2 can be resolved, the relative peak height/intensity ratio R = I delta'/I delta is greater in DPPC than in DHPC, suggesting that this parameter may be useful as a test of interdigitation.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bittman R., Clejan S., Jain M. K., Deroo P. W., Rosenthal A. F. Effects of sterols on permeability and phase transitions of bilayers from phosphatidylcholines lacking acyl groups. Biochemistry. 1981 May 12;20(10):2790–2795. doi: 10.1021/bi00513a013. [DOI] [PubMed] [Google Scholar]
- Boggs J. M., Stamp D., Hughes D. W., Deber C. M. Influence of ether linkage on the lamellar to hexagonal phase transition of ethanolamine phospholipids. Biochemistry. 1981 Sep 29;20(20):5728–5735. doi: 10.1021/bi00523a015. [DOI] [PubMed] [Google Scholar]
- Cameron D. G., Casal H. L., Gudgin E. F., Mantsch H. H. The gel phase of dipalmitoyl phosphatidylcholine. An infrared characterization of the acyl chain packing. Biochim Biophys Acta. 1980 Mar 13;596(3):463–467. doi: 10.1016/0005-2736(80)90135-2. [DOI] [PubMed] [Google Scholar]
- Cameron D. G., Casal H. L., Mantsch H. H. Characterization of the pretransition in 1,2-dipalmitoyl-sn-glycero-3-phosphocholine by Fourier transform infrared spectroscopy. Biochemistry. 1980 Aug 5;19(16):3665–3672. doi: 10.1021/bi00557a005. [DOI] [PubMed] [Google Scholar]
- Casal H. L., Mantsch H. H. Polymorphic phase behaviour of phospholipid membranes studied by infrared spectroscopy. Biochim Biophys Acta. 1984 Dec 4;779(4):381–401. doi: 10.1016/0304-4157(84)90017-0. [DOI] [PubMed] [Google Scholar]
- Fischmeister I. Infrared absorption spectroscopy of normal and substituted long-chain fatty acids and esters in the solid state. Prog Chem Fats Other Lipids. 1975;14(3):91–162. doi: 10.1016/0079-6832(75)90003-8. [DOI] [PubMed] [Google Scholar]
- Friedberg S. J., Halpert M. Ehrlich ascites tumor cell surface membranes: an abnormality in ether lipid content. J Lipid Res. 1978 Jan;19(1):57–64. [PubMed] [Google Scholar]
- Fringeli U. P. The structure of lipids and proteins studied by attenuated total reflection (ATR) infrared spectroscopy. II. Oriented layers of a homologous series: phosphatidylethanolamine to phosphatidylcholine. Z Naturforsch C. 1977 Jan-Feb;32(1-2):20–45. doi: 10.1515/znc-1977-1-205. [DOI] [PubMed] [Google Scholar]
- Hauser H., Pascher I., Sundell S. Conformation of phospholipids. Crystal structure of a lysophosphatidylcholine analogue. J Mol Biol. 1980 Mar 5;137(3):249–264. doi: 10.1016/0022-2836(80)90315-0. [DOI] [PubMed] [Google Scholar]
- Huang C., Mason J. T., Stephenson F. A., Levin I. W. Polymorphic phase behavior of platelet-activating factor. Biophys J. 1986 Mar;49(3):587–595. doi: 10.1016/S0006-3495(86)83686-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui S. W., Huang C. H. X-ray diffraction evidence for fully interdigitated bilayers of 1-stearoyllysophosphatidylcholine. Biochemistry. 1986 Mar 25;25(6):1330–1335. doi: 10.1021/bi00354a021. [DOI] [PubMed] [Google Scholar]
- Jain M. K., Crecely R. W., Hille J. D., de Haas G. H., Gruner S. M. Phase properties of the aqueous dispersions of n-octadecylphosphocholine. Biochim Biophys Acta. 1985 Feb 28;813(1):68–76. doi: 10.1016/0005-2736(85)90346-3. [DOI] [PubMed] [Google Scholar]
- Lee T. C., Fitzgerald V. Phase transitions of alkyl ether analogs of phosphatidylcholine. Biochim Biophys Acta. 1980 May 8;598(1):189–192. doi: 10.1016/0005-2736(80)90278-3. [DOI] [PubMed] [Google Scholar]
- Levin I. W., Keihn E., Harris W. C. A Raman spectroscopic study on the effect of cholesterol on lipid packing in diether phosphatidylcholine bilayer dispersions. Biochim Biophys Acta. 1985 Oct 24;820(1):40–47. doi: 10.1016/0005-2736(85)90213-5. [DOI] [PubMed] [Google Scholar]
- Levin I. W., Thompson T. E., Barenholz Y., Huang C. Two types of hydrocarbon chain interdigitation in sphingomyelin bilayers. Biochemistry. 1985 Oct 22;24(22):6282–6286. doi: 10.1021/bi00343a036. [DOI] [PubMed] [Google Scholar]
- Lewis E. N., Bittman R., Levin I. W. Methyl group substitution at C(1), C(2) or C(3) of the glycerol backbone of a diether phosphocholine: a comparative study of bilayer chain disorder in the gel and liquid-crystalline phases. Biochim Biophys Acta. 1986 Sep 25;861(1):44–52. doi: 10.1016/0005-2736(86)90369-x. [DOI] [PubMed] [Google Scholar]
- Mushayakarara E. C., Mantsch H. H. Thermotropic phase behavior of the platelet-activating factor: an infrared spectroscopic study. Can J Biochem Cell Biol. 1985 Oct;63(10):1071–1076. doi: 10.1139/o85-133. [DOI] [PubMed] [Google Scholar]
- O'Leary T. J., Levin I. W. Raman spectroscopic study of an interdigitated lipid bilayer. Dipalmitoylphosphatidylcholine dispersed in glycerol. Biochim Biophys Acta. 1984 Oct 3;776(2):185–189. doi: 10.1016/0005-2736(84)90207-4. [DOI] [PubMed] [Google Scholar]
- Paltauf F., Hauser H., Phillips M. C. Monolayer characteristics of some 1,2-diacyl, I-alkyl-2-acyl and 1,2-dialkyl phospholipids at the air-water interface. Biochim Biophys Acta. 1971 Dec 3;249(2):539–547. doi: 10.1016/0005-2736(71)90129-5. [DOI] [PubMed] [Google Scholar]
- Pugh E. L., Kates M., Hanahan D. J. Characterization of the alkyl ether species of phosphatidylcholine in bovine heart. J Lipid Res. 1977 Nov;18(6):710–716. [PubMed] [Google Scholar]
- Ruocco M. J., Makriyannis A., Siminovitch D. J., Griffin R. G. Deuterium NMR investigation of ether- and ester-linked phosphatidylcholine bilayers. Biochemistry. 1985 Aug 27;24(18):4844–4851. doi: 10.1021/bi00339a018. [DOI] [PubMed] [Google Scholar]
- Ruocco M. J., Siminovitch D. J., Griffin R. G. Comparative study of the gel phases of ether- and ester-linked phosphatidylcholines. Biochemistry. 1985 May 7;24(10):2406–2411. doi: 10.1021/bi00331a003. [DOI] [PubMed] [Google Scholar]
- Schwartz F. T., Paltauf F. Studies on the interaction of cholesterol with diester- and dietherlecithin. Chem Phys Lipids. 1976 Nov;17(4):423–434. doi: 10.1016/0009-3084(76)90044-x. [DOI] [PubMed] [Google Scholar]
- Seddon J. M., Cevc G., Marsh D. Calorimetric studies of the gel-fluid (L beta-L alpha) and lamellar-inverted hexagonal (L alpha-HII) phase transitions in dialkyl- and diacylphosphatidylethanolamines. Biochemistry. 1983 Mar 1;22(5):1280–1289. doi: 10.1021/bi00274a045. [DOI] [PubMed] [Google Scholar]
- Serrallach E. N., Dijkman R., de Haas G. H., Shipley G. G. Structure and thermotropic properties of 1,3-dipalmitoyl-glycero-2-phosphocholine. J Mol Biol. 1983 Oct 15;170(1):155–174. doi: 10.1016/s0022-2836(83)80231-9. [DOI] [PubMed] [Google Scholar]
- Siminovitch D. J., Jeffrey K. R., Eibl H. A comparison of the headgroup conformation and dynamics in synthetic analogs of dipalmitoylphosphatidylcholine. Biochim Biophys Acta. 1983 Jan 5;727(1):122–134. doi: 10.1016/0005-2736(83)90376-0. [DOI] [PubMed] [Google Scholar]
- Vaughan D. J., Keough K. M. Changes in phase transitions of phosphatidylethanolamine- and phosphatidylcholine-water dispersions induced by small modifications in the headgroup and backbone regions. FEBS Lett. 1974 Oct 1;47(1):158–161. doi: 10.1016/0014-5793(74)80449-7. [DOI] [PubMed] [Google Scholar]
- Wong P. T. Raman spectroscopy of thermotropic and high-pressure phases of aqueous phospholipid dispersions. Annu Rev Biophys Bioeng. 1984;13:1–24. doi: 10.1146/annurev.bb.13.060184.000245. [DOI] [PubMed] [Google Scholar]


