Abstract
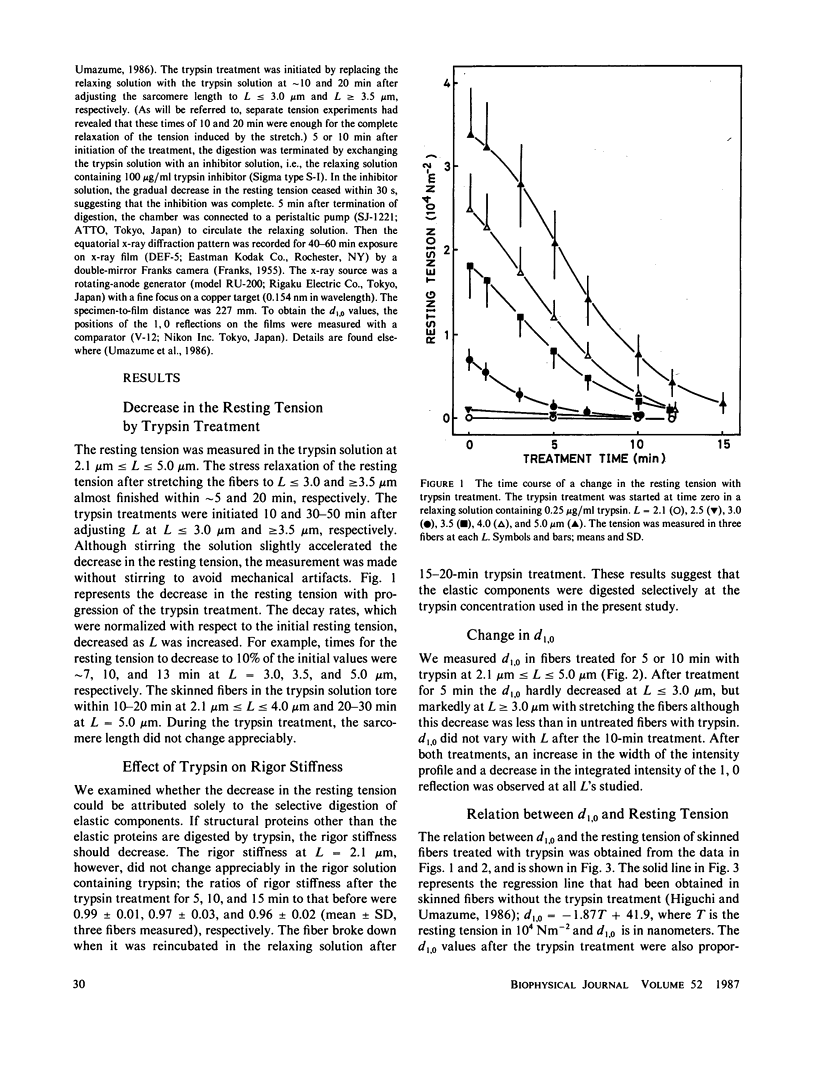
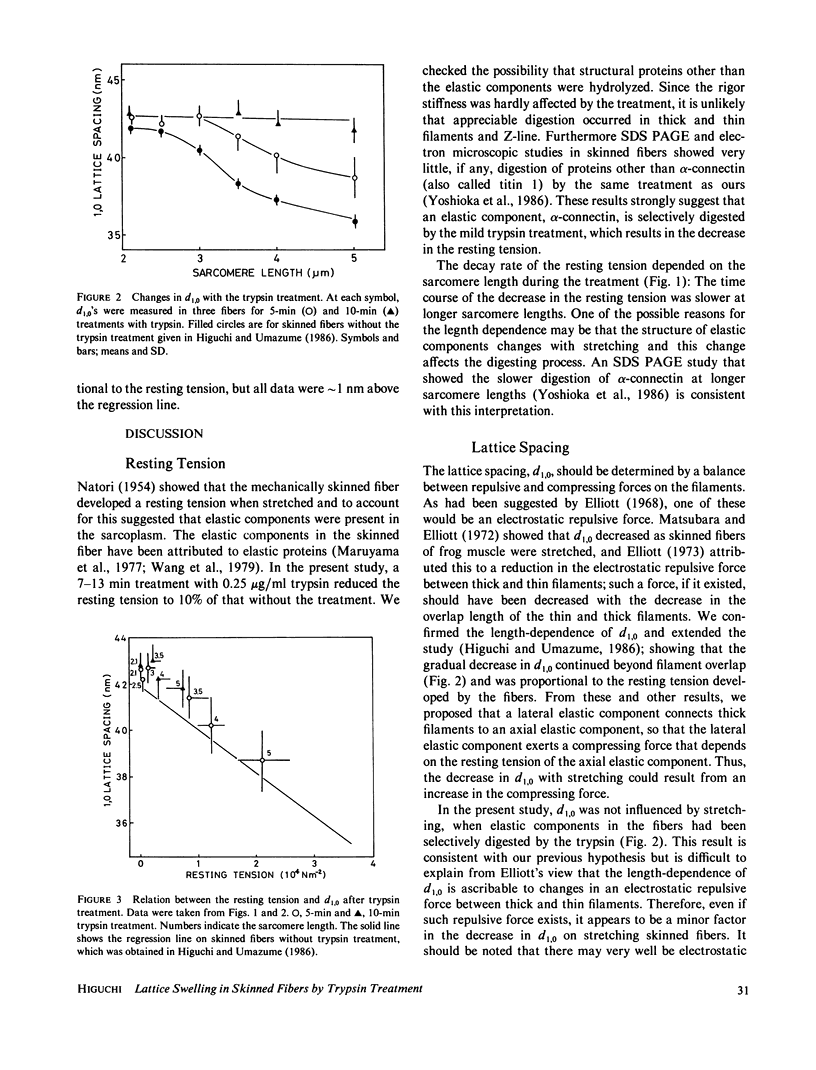
Changes in the 1.0 lattice spacing during trypsin (0.25 micrograms/ml) treatment in mechanically skinned single fibers of frog muscle was examined by an x-ray diffraction method at various sarcomere lengths. The resting tension of a relaxed fiber was decreased by trypsin treatment but the stiffness of a rigor fiber was not, suggesting that elastic components were selectively digested. With progression of the digestion, the lattice spacing increased remarkably at longer sarcomere lengths and finally became independent of the sarcomere length. The increase in the lattice spacing was proportional to the decrease in the resting tension. These results support our previous suggestion (Higuchi, H., and Y. Umazume, 1986, Biophys. J., 50:385-389) that the lattice spacing decreases at long lengths due to compressive force exerted by a lateral elastic component that connects thick filaments to an axial elastic component. Consequently, it is unlikely that the decrease in the lattice spacing is determined by a decrease in the repulsive force between thick and thin filaments with stretching a fiber.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Elliott G. F. Donnan and osmotic effects in muscle fibres without membranes. J Mechanochem Cell Motil. 1973 May;2(1):83–89. [PubMed] [Google Scholar]
- Elliott G. F. Force-balances and stability in hexagonally-packed polyelectrolyte systems. J Theor Biol. 1968 Oct;21(1):71–87. doi: 10.1016/0022-5193(68)90060-x. [DOI] [PubMed] [Google Scholar]
- Higuchi H., Umazume Y. Lattice shrinkage with increasing resting tension in stretched, single skinned fibers of frog muscle. Biophys J. 1986 Sep;50(3):385–389. doi: 10.1016/S0006-3495(86)83474-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi H., Umazume Y. Localization of the parallel elastic components in frog skinned muscle fibers studied by the dissociation of the A- and I-bands. Biophys J. 1985 Jul;48(1):137–147. doi: 10.1016/S0006-3495(85)83767-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowits R., Kempner E. S., Bisher M. E., Podolsky R. J. A physiological role for titin and nebulin in skeletal muscle. Nature. 1986 Sep 11;323(6084):160–164. doi: 10.1038/323160a0. [DOI] [PubMed] [Google Scholar]
- Maruyama K., Matsubara S., Natori R., Nonomura Y., Kimura S. Connectin, an elastic protein of muscle. Characterization and Function. J Biochem. 1977 Aug;82(2):317–337. [PubMed] [Google Scholar]
- Matsubara I., Elliott G. F. X-ray diffraction studies on skinned single fibres of frog skeletal muscle. J Mol Biol. 1972 Dec 30;72(3):657–669. doi: 10.1016/0022-2836(72)90183-0. [DOI] [PubMed] [Google Scholar]
- Umazume Y., Onodera S., Higuchi H. Width and lattice spacing in radially compressed frog skinned muscle fibres at various pH values, magnesium ion concentrations and ionic strengths. J Muscle Res Cell Motil. 1986 Jun;7(3):251–258. doi: 10.1007/BF01753558. [DOI] [PubMed] [Google Scholar]
- Wang K., McClure J., Tu A. Titin: major myofibrillar components of striated muscle. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3698–3702. doi: 10.1073/pnas.76.8.3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Ramirez-Mitchell R. A network of transverse and longitudinal intermediate filaments is associated with sarcomeres of adult vertebrate skeletal muscle. J Cell Biol. 1983 Feb;96(2):562–570. doi: 10.1083/jcb.96.2.562. [DOI] [PMC free article] [PubMed] [Google Scholar]


