Abstract
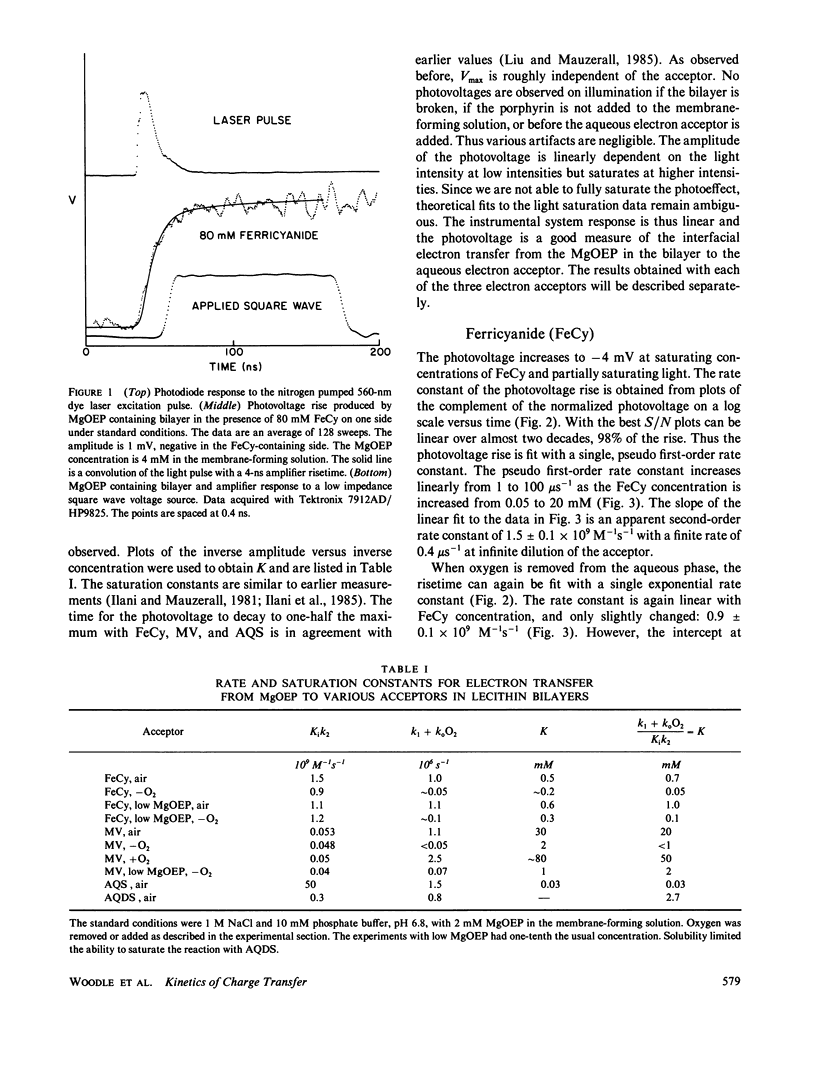
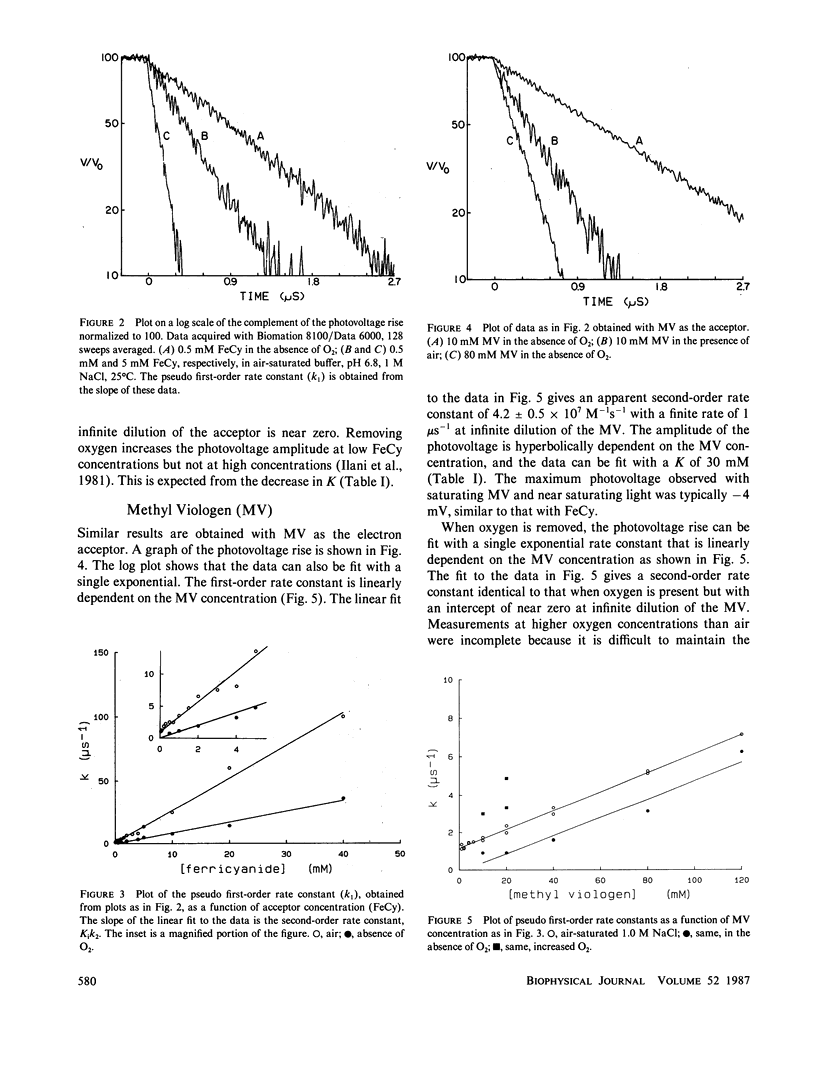
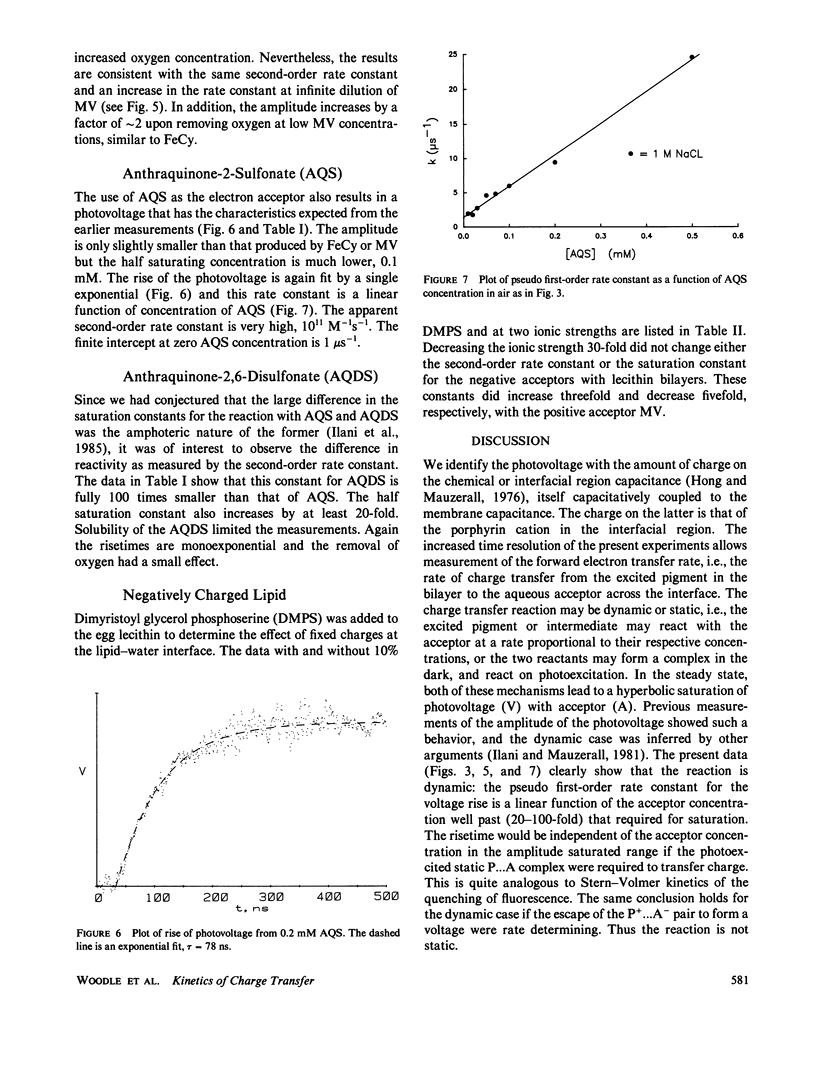
Advances in instrumentation allow electrical measurements across the planar lipid bilayer to be made with nanosecond time resolution. The electron transfer reaction between photoexcited magnesium octaethylporphyrin in the lipid to a variety of ionically charged acceptors in the water is found to be purely dynamic over a wide range of concentrations of acceptors and up to the time constant of the apparatus, 4 ns. The saturation of the amplitude of the photovoltage with increasing concentration of acceptor is caused by the finite lifetime of the excited state, not by formation of a static pigment-acceptor complex. The reactions are an excellent probe of the lipid-water interface over an extended time scale. No appreciable barrier to reaction exists at this interface beyond the 5-ns time. That is, any water or choline group structure may be evanescent on this time scale. Electrostatic interactions indicate that the acceptor molecules penetrate to the level of the phosphocholine groups with differing orientations. It will be possible to extend the time scale into the picosecond range by decreasing the response time and by deconvolutions.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carapellucci P. A., Mauzerall D. Photosynthesis and porphyrin excited state redox reactions. Ann N Y Acad Sci. 1975 Apr 15;244:214–238. doi: 10.1111/j.1749-6632.1975.tb41533.x. [DOI] [PubMed] [Google Scholar]
- Cherry R. J., Hsu K., Chapman D. Polarised absorption spectroscopy of chlorophyll-lipid membranes. Biochim Biophys Acta. 1972 Jun 23;267(3):512–522. doi: 10.1016/0005-2728(72)90179-x. [DOI] [PubMed] [Google Scholar]
- Davis J. H. The description of membrane lipid conformation, order and dynamics by 2H-NMR. Biochim Biophys Acta. 1983 Mar 21;737(1):117–171. doi: 10.1016/0304-4157(83)90015-1. [DOI] [PubMed] [Google Scholar]
- Flewelling R. F., Hubbell W. L. The membrane dipole potential in a total membrane potential model. Applications to hydrophobic ion interactions with membranes. Biophys J. 1986 Feb;49(2):541–552. doi: 10.1016/S0006-3495(86)83664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong F. T. Charge transfer across pigmented bilayer lipid membrane and its interfaces. Photochem Photobiol. 1976 Aug;24(2):155–189. doi: 10.1111/j.1751-1097.1976.tb06809.x. [DOI] [PubMed] [Google Scholar]
- Hong F. T., Mauzerall D. Interfacial photoreactions and chemical capacitance in lipid bilayers. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1564–1568. doi: 10.1073/pnas.71.4.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong F. T., Mauzerall D. Photoemf at a single membrane-solution interface specific to lipid bilayers containing magnesium porphyrins. Nat New Biol. 1972 Nov 29;240(100):154–155. doi: 10.1038/newbio240154a0. [DOI] [PubMed] [Google Scholar]
- Huang C. H., Charlton J. P. Interactions of phosphatidylcholine vesicles with 2-p-toluidinylnaphthalene-6-sulfonate. Biochemistry. 1972 Feb 29;11(5):735–740. doi: 10.1021/bi00755a010. [DOI] [PubMed] [Google Scholar]
- Ilani A., Liu T. M., Mauzerall D. The effect of oxygen on the amplitude of photodriven electron transfer across the lipid bilayer-water interface. Biophys J. 1985 May;47(5):679–684. doi: 10.1016/S0006-3495(85)83964-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilani A., Mauzerall D. The potential span of photoredox reactions of porphyrins and chlorophyll at the lipid bilayer-water interface. Biophys J. 1981 Jul;35(1):79–92. doi: 10.1016/S0006-3495(81)84775-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T. M., Mauzerall D. Distributed kinetics of decay of the photovoltage at the lipid bilayer-water interface. Biophys J. 1985 Jul;48(1):1–7. doi: 10.1016/S0006-3495(85)83755-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauzerall D. Electron transfer reactions and photoexcited porphyrins. Brookhaven Symp Biol. 1976 Jun 7;(28):64–73. [PubMed] [Google Scholar]
- Raudino A., Mauzerall D. Dielectric properties of the polar head group region of zwitterionic lipid bilayers. Biophys J. 1986 Sep;50(3):441–449. doi: 10.1016/S0006-3495(86)83480-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. L., Oldfield E. Dynamic structure of membranes by deuterium NMR. Science. 1984 Jul 20;225(4659):280–288. doi: 10.1126/science.6740310. [DOI] [PubMed] [Google Scholar]
- Varnadore W. E., Jr, Arrieta R. T., Duchek J. R., Huebner J. S. Erythrosin and pH gradient induced photo-voltages in bilayer membranes. J Membr Biol. 1982;65(1-2):147–153. doi: 10.1007/BF01870478. [DOI] [PubMed] [Google Scholar]
- Watts A., Poile T. W. Direct determination by 2H-NMR of the ionization state of phospholipids and of a local anaesthetic at the membrane surface. Biochim Biophys Acta. 1986 Oct 9;861(2):368–372. doi: 10.1016/0005-2736(86)90440-2. [DOI] [PubMed] [Google Scholar]
- Woodle M. C., Mauzerall D. Photoinitiated ion movements in bilayer membranes containing magnesium octaethylporphyrin. Biophys J. 1986 Sep;50(3):431–439. doi: 10.1016/S0006-3495(86)83479-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


