Abstract
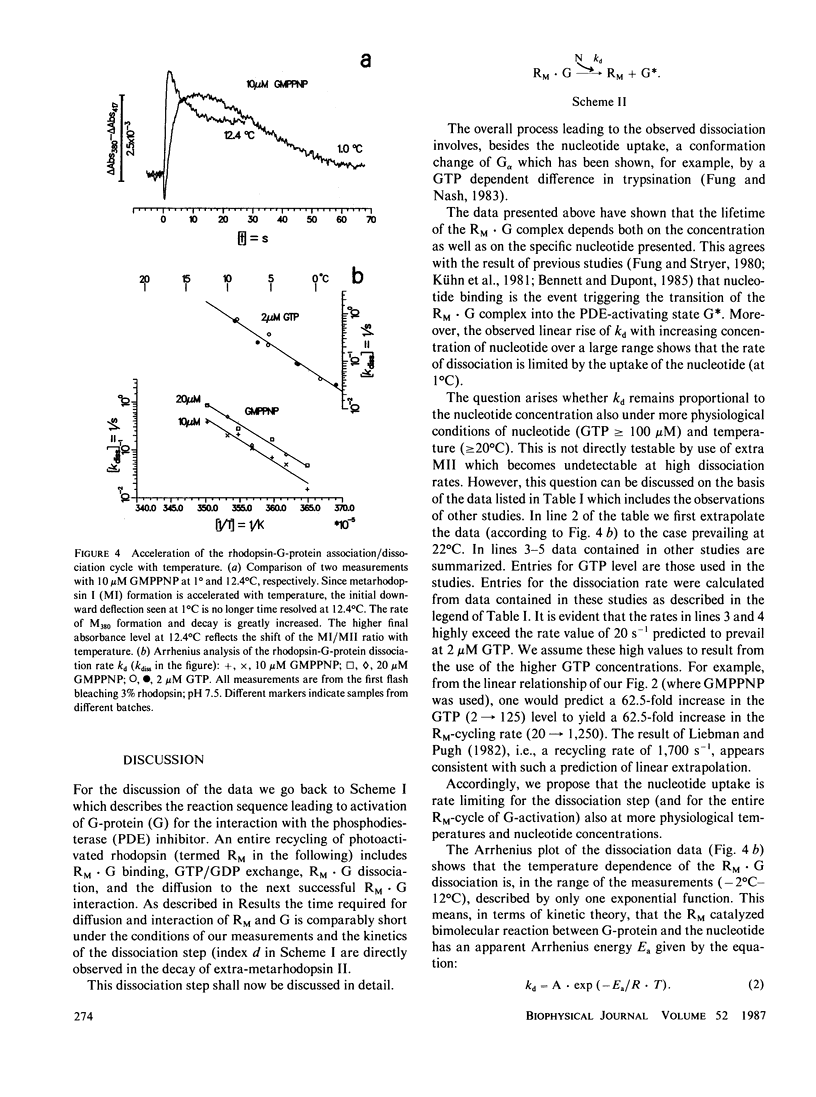
The thermal activation barrier of guanosine triphosphate dependent dissociation of the light-induced rhodopsin-G-protein complex has been determined using a spectroscopic technique (enhanced formation of metarhodopsin II). The dissociation rate has been measured in the range - 2 degrees C less than or equal to t less than or equal to 12 degrees C. The Arrhenius plot yields apparent activation energies: 166 +/- 10 kJmol-1 with 5'-guanylylimidodiphosphate (GMPPNP) and 175 +/- 15 kJmol-1 with GTP. The rhodopsin-G-protein dissociation rate is linearly related to the concentration of GMPPNP in the measurable range (less than or equal to 200 microM). The data show that, at low temperature (1 degree C), the rate limiting step of G-protein activation is the bimolecular reaction between the protein and the nucleotide. This also seems to hold true for more physiological conditions as suggested by extrapolation and comparison with nucleotide exchange rates in the literature. The high activation barrier of the nucleotide exchange reaction is explained in terms of rapid endothermic preequilibrium between an inactive and an exchanging state of the rhodopsin-G-protein complex.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett N., Dupont Y. The G-protein of retinal rod outer segments (transducin). Mechanism of interaction with rhodopsin and nucleotides. J Biol Chem. 1985 Apr 10;260(7):4156–4168. [PubMed] [Google Scholar]
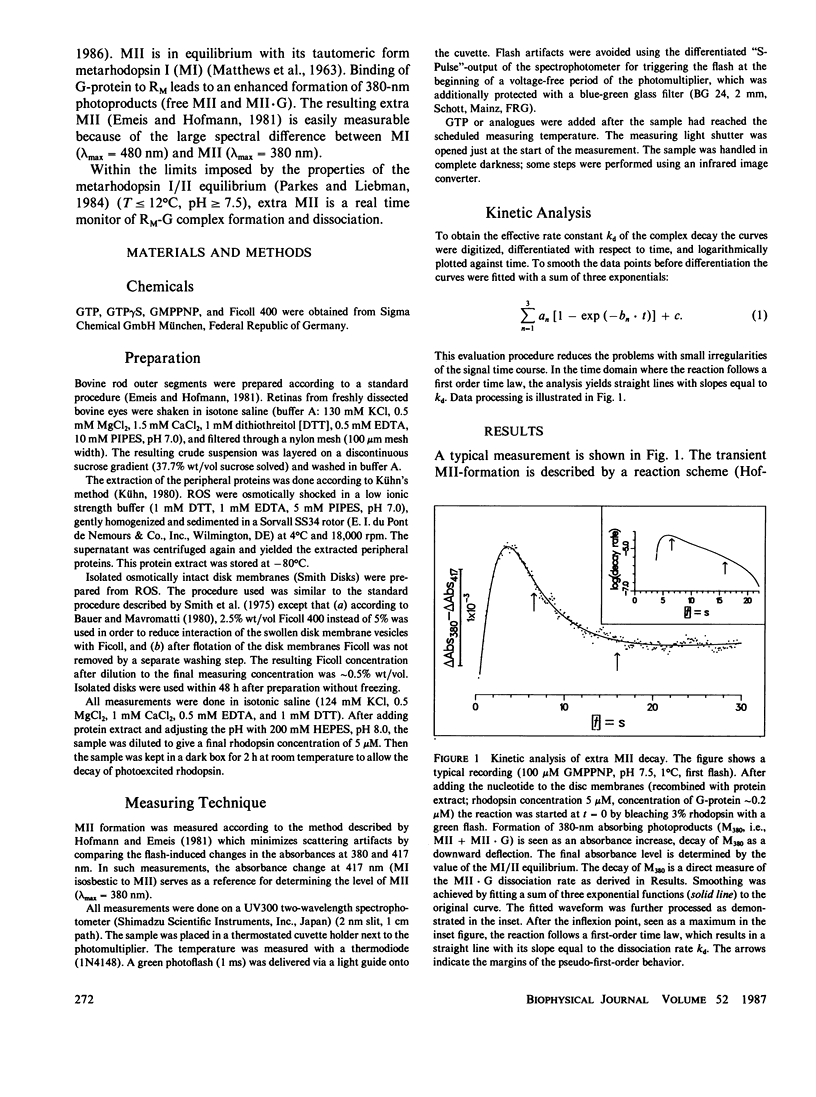
- Emeis D., Hofmann K. P. Shift in the relation between flash-induced metarhodopsin I and metarhodpsin II within the first 10% rhodopsin bleaching in bovine disc membranes. FEBS Lett. 1981 Dec 28;136(2):201–207. doi: 10.1016/0014-5793(81)80618-7. [DOI] [PubMed] [Google Scholar]
- Emeis D., Kühn H., Reichert J., Hofmann K. P. Complex formation between metarhodopsin II and GTP-binding protein in bovine photoreceptor membranes leads to a shift of the photoproduct equilibrium. FEBS Lett. 1982 Jun 21;143(1):29–34. doi: 10.1016/0014-5793(82)80266-4. [DOI] [PubMed] [Google Scholar]
- Fung B. K., Hurley J. B., Stryer L. Flow of information in the light-triggered cyclic nucleotide cascade of vision. Proc Natl Acad Sci U S A. 1981 Jan;78(1):152–156. doi: 10.1073/pnas.78.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung B. K., Nash C. R. Characterization of transducin from bovine retinal rod outer segments. II. Evidence for distinct binding sites and conformational changes revealed by limited proteolysis with trypsin. J Biol Chem. 1983 Sep 10;258(17):10503–10510. [PubMed] [Google Scholar]
- Godchaux W., 3rd, Zimmerman W. F. Membrane-dependent guanine nucleotide binding and GTPase activities of soluble protein from bovine rod cell outer segments. J Biol Chem. 1979 Aug 25;254(16):7874–7884. [PubMed] [Google Scholar]
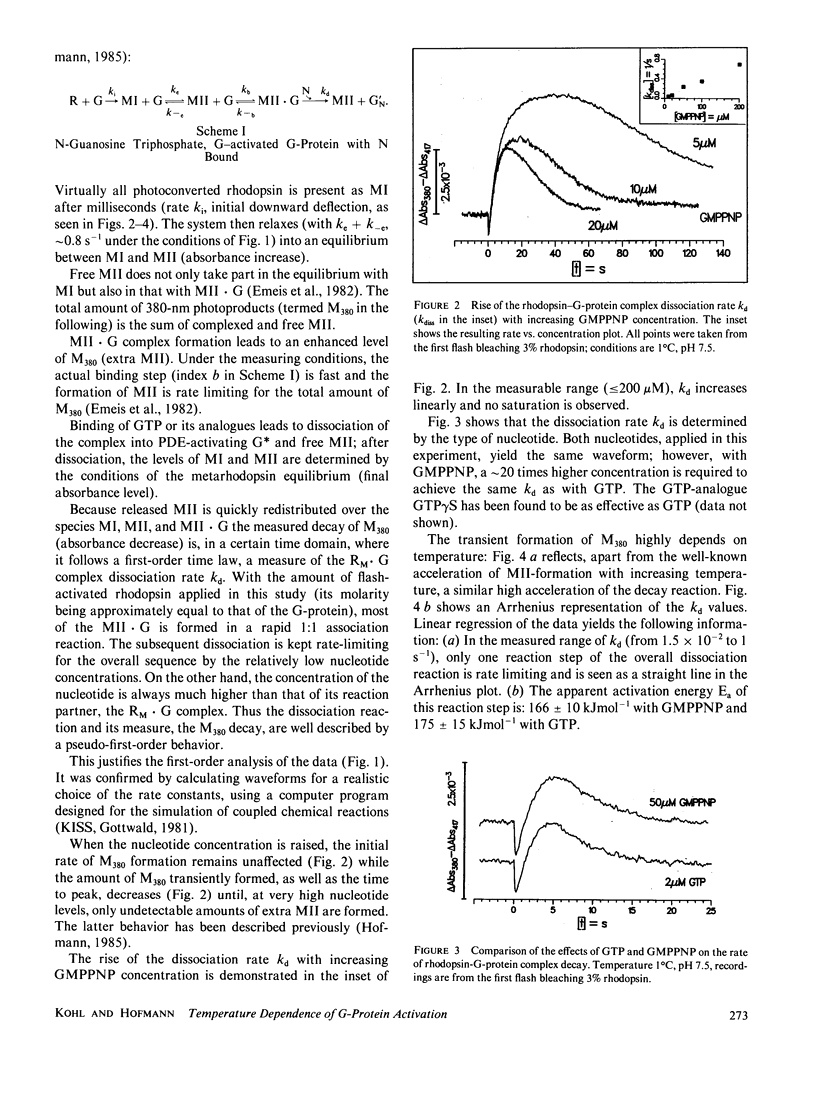
- Hofmann K. P. Effect of GTP on the rhodopsin-G-protein complex by transient formation of extra metarhodopsin II. Biochim Biophys Acta. 1985 Nov 27;810(2):278–281. doi: 10.1016/0005-2728(85)90143-4. [DOI] [PubMed] [Google Scholar]
- Kwok-Keung Fung B., Stryer L. Photolyzed rhodopsin catalyzes the exchange of GTP for bound GDP in retinal rod outer segments. Proc Natl Acad Sci U S A. 1980 May;77(5):2500–2504. doi: 10.1073/pnas.77.5.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn H., Bennett N., Michel-Villaz M., Chabre M. Interactions between photoexcited rhodopsin and GTP-binding protein: kinetic and stoichiometric analyses from light-scattering changes. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6873–6877. doi: 10.1073/pnas.78.11.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn H. Light- and GTP-regulated interaction of GTPase and other proteins with bovine photoreceptor membranes. Nature. 1980 Feb 7;283(5747):587–589. doi: 10.1038/283587a0. [DOI] [PubMed] [Google Scholar]
- Liebman P. A., Pugh E. N., Jr Gain, speed and sensitivity of GTP binding vs PDE activation in visual excitation. Vision Res. 1982;22(12):1475–1480. doi: 10.1016/0042-6989(82)90212-7. [DOI] [PubMed] [Google Scholar]
- Liebman P. A., Sitaramayya A. Role of G-protein-receptor interaction in amplified phosphodiesterase activation of retinal rods. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;17:215–225. [PubMed] [Google Scholar]
- Longstaff C., Calhoon R. D., Rando R. R. Deprotonation of the Schiff base of rhodopsin is obligate in the activation of the G protein. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4209–4213. doi: 10.1073/pnas.83.12.4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATTHEWS R. G., HUBBARD R., BROWN P. K., WALD G. TAUTOMERIC FORMS OF METARHODOPSIN. J Gen Physiol. 1963 Nov;47:215–240. doi: 10.1085/jgp.47.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkes J. H., Liebman P. A. Temperature and pH dependence of the metarhodopsin I-metarhodopsin II kinetics and equilibria in bovine rod disk membrane suspensions. Biochemistry. 1984 Oct 9;23(21):5054–5061. doi: 10.1021/bi00316a035. [DOI] [PubMed] [Google Scholar]
- Robinson P. R., Radeke M. J., Cote R. H., Bownds M. D. cGMP influences guanine nucleotide binding to frog photoreceptor G-protein. J Biol Chem. 1986 Jan 5;261(1):313–318. [PubMed] [Google Scholar]
- Schleicher A., Hofmann K. P. Kinetic study on the equilibrium between membrane-bound and free photoreceptor G-protein. J Membr Biol. 1987;95(3):271–281. doi: 10.1007/BF01869489. [DOI] [PubMed] [Google Scholar]
- Smith H. G., Jr, Stubbs G. W., Litman B. J. The isolation and purification of osmotically intact discs from retinal rod outer segments. Exp Eye Res. 1975 Mar;20(3):211–217. doi: 10.1016/0014-4835(75)90134-7. [DOI] [PubMed] [Google Scholar]
- Yamanaka G., Eckstein F., Stryer L. Interaction of retinal transducin with guanosine triphosphate analogues: specificity of the gamma-phosphate binding region. Biochemistry. 1986 Oct 7;25(20):6149–6153. doi: 10.1021/bi00368a048. [DOI] [PubMed] [Google Scholar]


