Abstract
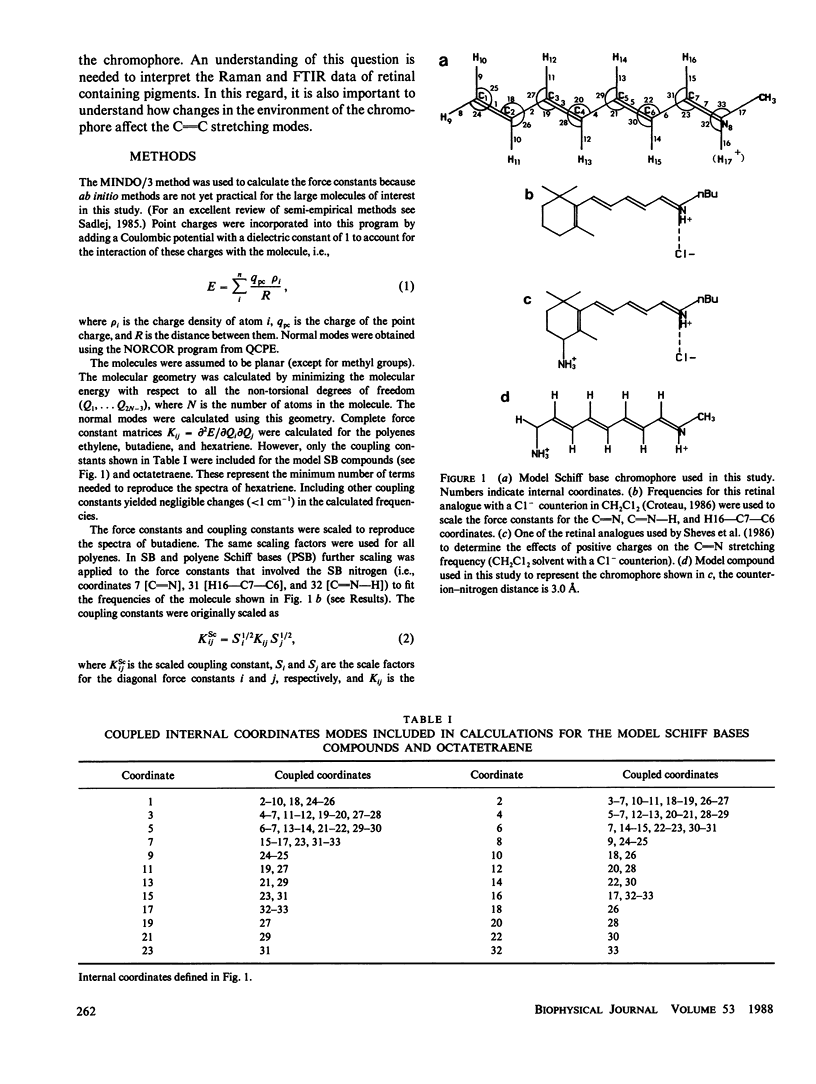
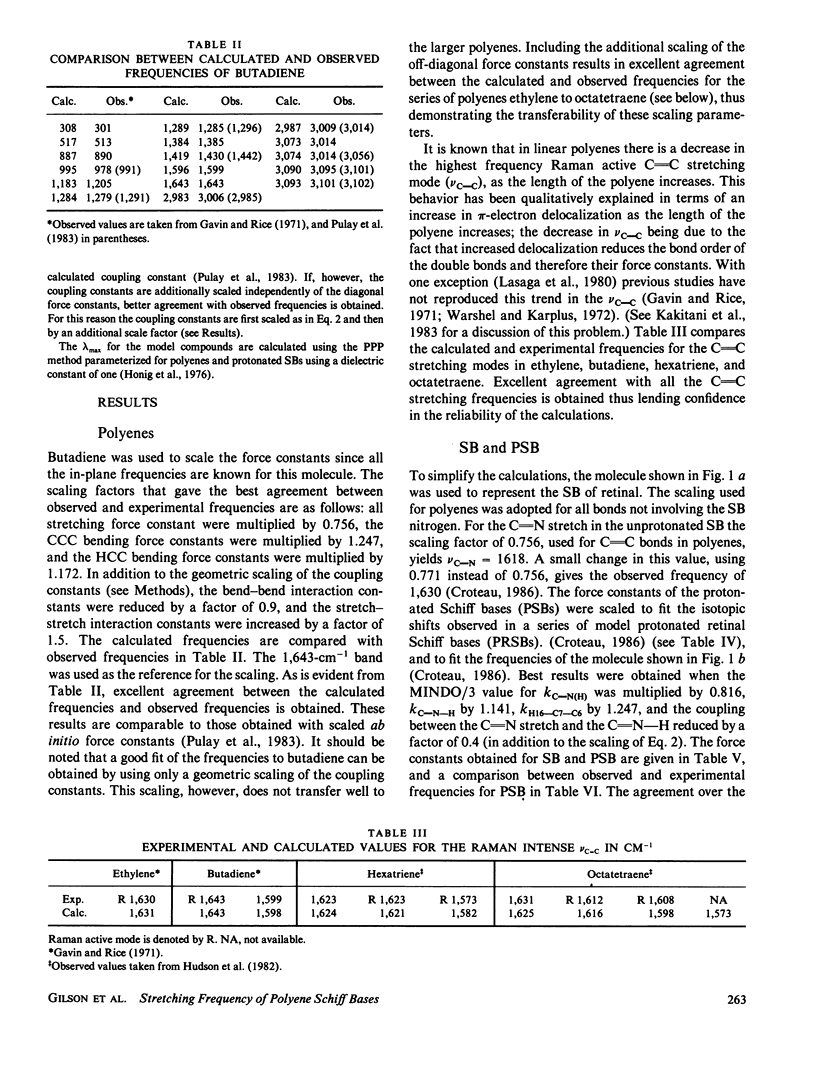
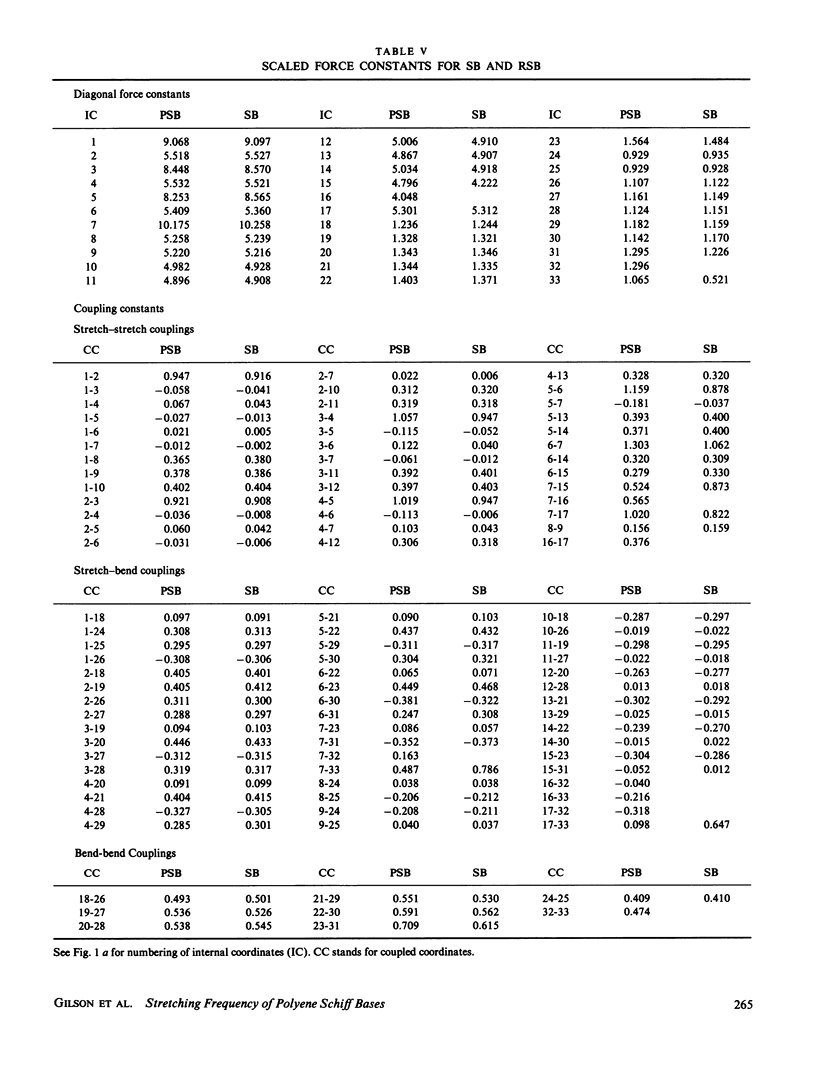
In this study quantum mechanical calculations of force constants and normal mode analysis are used to elucidate the factors that influence the C=C and C=N stretching frequencies in polyenes and in protonated Schiff bases. The C=N stretching frequency is found to depend on both the C=N stretching force constant and the C=N-H bending force constant. Due to the contributions of these two modes, the C=N stretching frequency is particularly sensitive to the magnitude of the Schiff base counterion interactions and to the hydrogen bonding environment of the Schiff base nitrogen. Models for chromophore-protein interactions in the retinal binding site and for the photochemical transformations of bacteriorhodopsin and rhodopsin are evaluated in light of these results.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Applebury M. L., Hargrave P. A. Molecular biology of the visual pigments. Vision Res. 1986;26(12):1881–1895. doi: 10.1016/0042-6989(86)90115-x. [DOI] [PubMed] [Google Scholar]
- Aton B., Doukas A. G., Callender R. H., Becher B., Ebrey T. G. Resonance Raman studies of the purple membrane. Biochemistry. 1977 Jun 28;16(13):2995–2999. doi: 10.1021/bi00632a029. [DOI] [PubMed] [Google Scholar]
- Aton B., Doukas A. G., Narva D., Callender R. H., Dinur U., Honig B. Resonance Raman studies of the primary photochemical event in visual pigments. Biophys J. 1980 Jan;29(1):79–94. doi: 10.1016/S0006-3495(80)85119-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baasov T., Friedman N., Sheves M. Factors affecting the C = N stretching in protonated retinal Schiff base: a model study for bacteriorhodopsin and visual pigments. Biochemistry. 1987 Jun 2;26(11):3210–3217. doi: 10.1021/bi00385a041. [DOI] [PubMed] [Google Scholar]
- Cooper A. Energy uptake in the first step of visual excitation. Nature. 1979 Nov 29;282(5738):531–533. doi: 10.1038/282531a0. [DOI] [PubMed] [Google Scholar]
- Harbison G. S., Smith S. O., Pardoen J. A., Courtin J. M., Lugtenburg J., Herzfeld J., Mathies R. A., Griffin R. G. Solid-state 13C NMR detection of a perturbed 6-s-trans chromophore in bacteriorhodopsin. Biochemistry. 1985 Nov 19;24(24):6955–6962. doi: 10.1021/bi00345a031. [DOI] [PubMed] [Google Scholar]
- Hargrave P. A., McDowell J. H., Curtis D. R., Wang J. K., Juszczak E., Fong S. L., Rao J. K., Argos P. The structure of bovine rhodopsin. Biophys Struct Mech. 1983;9(4):235–244. doi: 10.1007/BF00535659. [DOI] [PubMed] [Google Scholar]
- Hol W. G. The role of the alpha-helix dipole in protein function and structure. Prog Biophys Mol Biol. 1985;45(3):149–195. doi: 10.1016/0079-6107(85)90001-x. [DOI] [PubMed] [Google Scholar]
- Honig B., Ebrey T., Callender R. H., Dinur U., Ottolenghi M. Photoisomerization, energy storage, and charge separation: a model for light energy transduction in visual pigments and bacteriorhodopsin. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2503–2507. doi: 10.1073/pnas.76.6.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honig B., Greenberg A. D., Dinur U., Ebrey T. G. Visual-pigment spectra: implications of the protonation of the retinal Schiff base. Biochemistry. 1976 Oct 19;15(21):4593–4599. doi: 10.1021/bi00666a008. [DOI] [PubMed] [Google Scholar]
- Khorana H. G., Gerber G. E., Herlihy W. C., Gray C. P., Anderegg R. J., Nihei K., Biemann K. Amino acid sequence of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5046–5050. doi: 10.1073/pnas.76.10.5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovchinnikov Y. A., Abdulaev N. G., Feigina M. Y., Kiselev A. V., Lobanov N. A. The structural basis of the functioning of bacteriorhodopsin: an overview. FEBS Lett. 1979 Apr 15;100(2):219–224. doi: 10.1016/0014-5793(79)80338-5. [DOI] [PubMed] [Google Scholar]
- Rothschild K. J., Marrero H. Infrared evidence that the Schiff base of bacteriorhodopsin is protonated: bR570 and K intermediates. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4045–4049. doi: 10.1073/pnas.79.13.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich J. L., McCain D. A., Nakanishi K., Okabe M., Shimizu N., Rodman H., Honig B., Bogomolni R. A. Chromophore/protein interaction in bacterial sensory rhodopsin and bacteriorhodopsin. Biophys J. 1986 Feb;49(2):479–483. doi: 10.1016/S0006-3495(86)83657-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockburger M., Klusmann W., Gattermann H., Massig G., Peters R. Photochemical cycle of bacteriorhodopsin studied by resonance Raman spectroscopy. Biochemistry. 1979 Oct 30;18(22):4886–4900. doi: 10.1021/bi00589a017. [DOI] [PubMed] [Google Scholar]


