Abstract
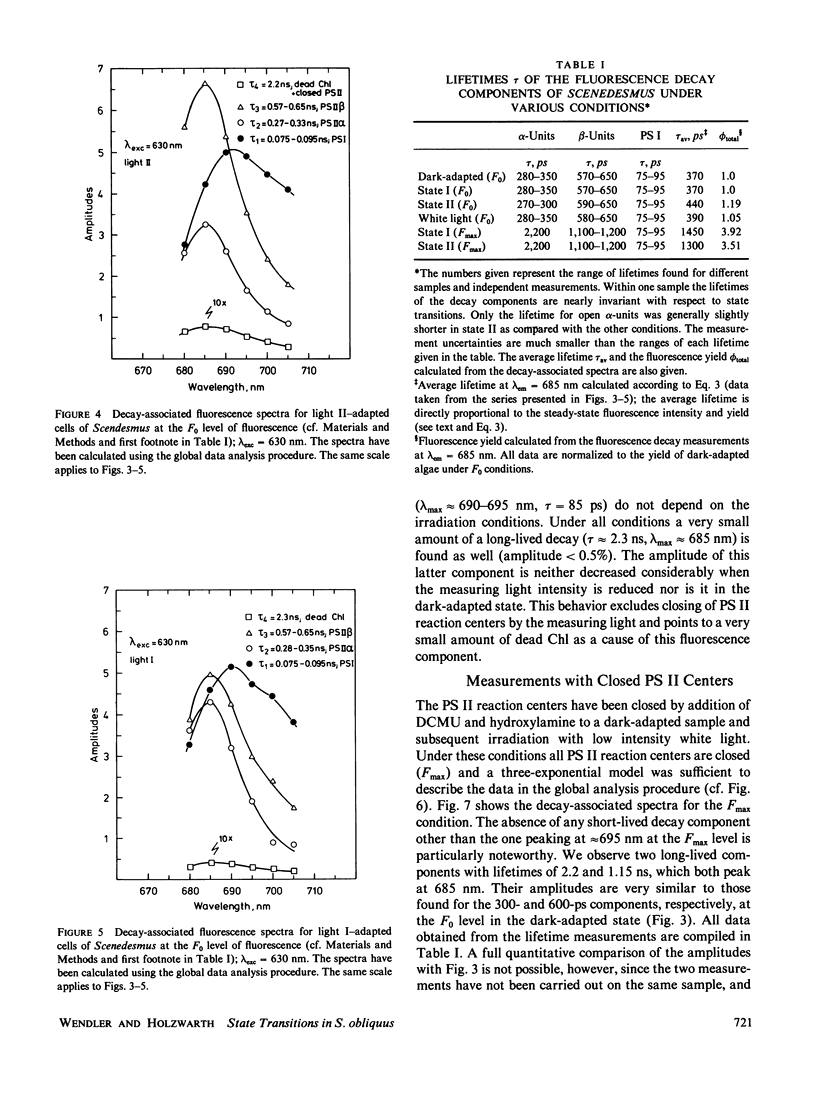
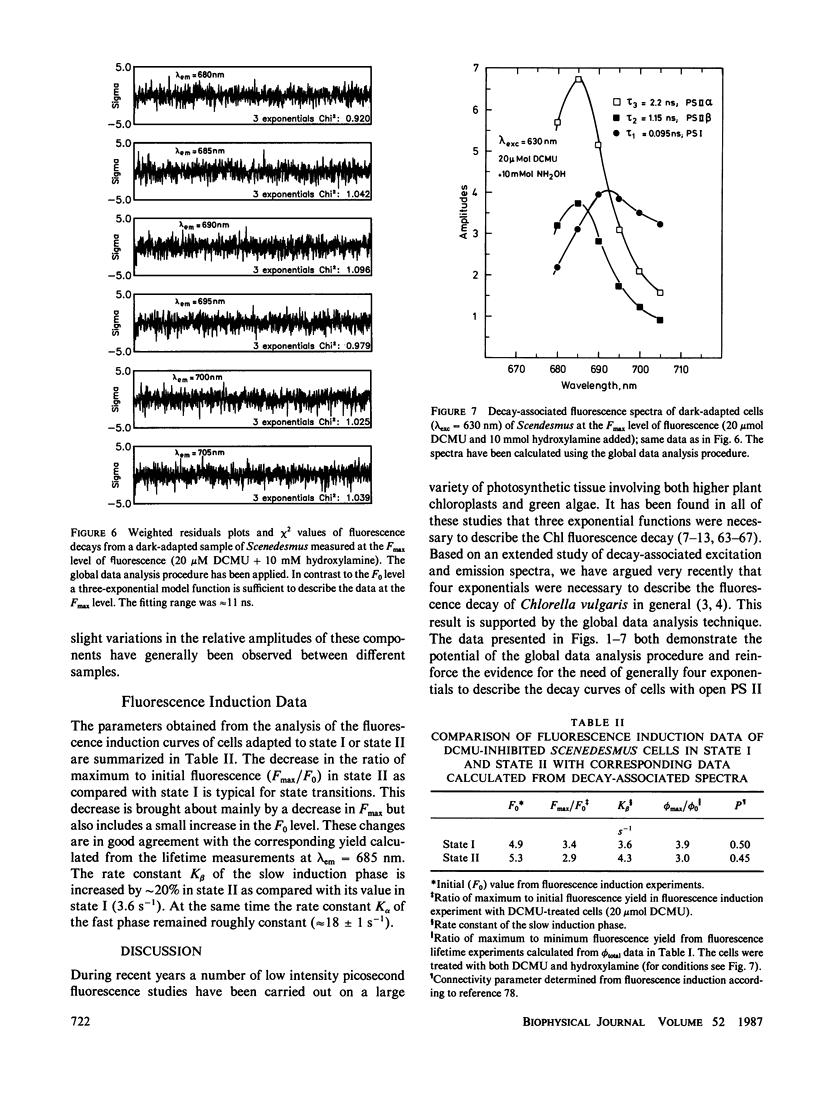
Decay-associated fluorescence spectra of the green alga Scenedesmus obliquus have been measured by single-photon timing with picosecond resolution in various states of light adaptation. The data have been analyzed by applying a global data analysis procedure. The amplitudes of the decay-associated spectra allow a determination of the relative antenna sizes of the photosystems. We arrive at the following conclusions: (a) The fluorescence kinetics of algal cells with open PS II centers (F0 level) have to be described by a sum of three exponential components. These decay components are attributed to photosystem (PS) I (τ ≈ 85 ps, λmaxem ≈ 695-700 nm), open PS II α-centers (τ ≈ 300 ps, λmaxem = 685 nm), and open PS II β-centers (τ ≈ 600 ps, λmaxem = 685 nm). A fourth component of very low amplitude (τ ≈ 2.2-2.3 ns, λmaxem = 685 nm) derives from dead chlorophyll. (b) At the Fmax level of fluorescence there are also three decay components. They originate from PS I with properties identical to those at the F0 level, from closed PS II α-centers (τ ≈ 2.2 ns, λmaxem = 685 nm) and from closed PS β-centers (τ ≈ 1.2 ns, λmaxem = 685 nm). (c) The major effect of light-induced state transitions on the fluorescence kinetics involves a change in the relative antenna size of α- and β-units brought about by the reversible migration of light-harvesting complexes between α-centers and β-centers. (d) A transition to state II does not measurably increase the direct absorption cross-section (antenna size) of PS I. Our data can be rationalized in terms of a model of the antenna organization that relates the effects of state transitions and light-harvesting complex phosphorylation with the concepts of PS II α,β-heterogeneity. We discuss why our results are in disagreement with those of a recent lifetime study of Chlorella by M. Hodges and I. Moya (1986, Biochim. Biophys. Acta., 849:193-202).
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. M., Melis A. Localization of different photosystems in separate regions of chloroplast membranes. Proc Natl Acad Sci U S A. 1983 Feb;80(3):745–749. doi: 10.1073/pnas.80.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J. Chloroplast phosphoproteins. The protein kinase of thylakoid membranes is light-dependent. FEBS Lett. 1979 Jul 15;103(2):342–344. doi: 10.1016/0014-5793(79)81358-7. [DOI] [PubMed] [Google Scholar]
- Bennett J., Steinback K. E., Arntzen C. J. Chloroplast phosphoproteins: regulation of excitation energy transfer by phosphorylation of thylakoid membrane polypeptides. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5253–5257. doi: 10.1073/pnas.77.9.5253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaventura C., Myers J. Fluorescence and oxygen evolution from Chlorella pyrenoidosa. Biochim Biophys Acta. 1969;189(3):366–383. doi: 10.1016/0005-2728(69)90168-6. [DOI] [PubMed] [Google Scholar]
- Butler W. L., Magde D., Berens S. J. Fluorescence lifetimes in the bipartite model of the photosynthetic apparatus with alpha, beta heterogeneity in photosystem II. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7510–7514. doi: 10.1073/pnas.80.24.7510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canaani O., Barber J., Malkin S. Evidence that phosphorylation and dephosphorylation regulate the distribution of excitation energy between the two photosystems of photosynthesis in vivo: Photoacoustic and fluorimetric study of an intact leaf. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1614–1618. doi: 10.1073/pnas.81.6.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulotty R. J., Mets L., Alberte R. S., Fleming G. R. Picosecond fluorescence study of photosynthetic mutants of Chlamydomonas reinhardii: origin of the fluorescence decay kinetics of chloroplasts. Photochem Photobiol. 1985 Apr;41(4):487–496. doi: 10.1111/j.1751-1097.1985.tb03516.x. [DOI] [PubMed] [Google Scholar]
- Haworth P. Protein phosphorylation-induced State I-State II transitions are dependent on thylakoid membrane microviscosity. Arch Biochem Biophys. 1983 Oct 1;226(1):145–154. doi: 10.1016/0003-9861(83)90279-5. [DOI] [PubMed] [Google Scholar]
- Hodges M., Barber J. State 1-State 2 Transitions in a Unicellular Green Algae : Analysis of In Vivo Chlorophyll Fluorescence Induction Curves in the Presence of 3-(3,4-Dichlorophenyl)-1, 1-dimethylurea (DCMU). Plant Physiol. 1983 Aug;72(4):1119–1122. doi: 10.1104/pp.72.4.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzwarth A. R., Wendler J., Suter G. W. Studies on Chromophore Coupling in Isolated Phycobiliproteins: II. Picosecond Energy Transfer Kinetics and Time-Resolved Fluorescence Spectra of C-Phycocyanin from Synechococcus 6301 as a Function of the Aggregation State. Biophys J. 1987 Jan;51(1):1–12. doi: 10.1016/S0006-3495(87)83306-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton P., Croze E. Characterization of two quenchers of chlorophyll fluorescence with different midpoint oxidation-reduction potentials in chloroplasts. Biochim Biophys Acta. 1979 Jan 11;545(1):188–201. doi: 10.1016/0005-2728(79)90125-7. [DOI] [PubMed] [Google Scholar]
- Karukstis K. K., Sauer K. Fluorescence decay kinetics of chlorophyll in photosynthetic membranes. J Cell Biochem. 1983;23(1-4):131–158. doi: 10.1002/jcb.240230112. [DOI] [PubMed] [Google Scholar]
- Kyle D. J., Staehelin L. A., Arntzen C. J. Lateral mobility of the light-harvesting complex in chloroplast membranes controls excitation energy distribution in higher plants. Arch Biochem Biophys. 1983 Apr 15;222(2):527–541. doi: 10.1016/0003-9861(83)90551-9. [DOI] [PubMed] [Google Scholar]
- Lavorel J., Joliot P. A connected model of the photosynthetic unit. Biophys J. 1972 Jul;12(7):815–831. doi: 10.1016/S0006-3495(72)86125-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley A. C., Butler W. L. Energy distribution in the photochemical apparatus of Porphyridium cruentum in state I and state II. Biochim Biophys Acta. 1980 Sep 5;592(2):349–363. doi: 10.1016/0005-2728(80)90195-4. [DOI] [PubMed] [Google Scholar]
- Melis A. Oxidation-reduction potential dependence of the two kinetic components in chloroplast system II primary photochemistry. FEBS Lett. 1978 Nov 15;95(2):202–206. doi: 10.1016/0014-5793(78)80993-4. [DOI] [PubMed] [Google Scholar]
- Melis A., Thielen A. P. The relative absorption cross-sections of photosystem I and photosystem II in chloroplasts from three types of Nicotiana tabacum. Biochim Biophys Acta. 1980 Feb 8;589(2):275–286. doi: 10.1016/0005-2728(80)90044-4. [DOI] [PubMed] [Google Scholar]
- Murata N. Control of excitation transfer in photosynthesis. I. Light-induced change of chlorophyll a fluorescence in Porphyridium cruentum. Biochim Biophys Acta. 1969 Feb 25;172(2):242–251. doi: 10.1016/0005-2728(69)90067-x. [DOI] [PubMed] [Google Scholar]
- Ried A., Reinhardt B. Distribution of excitation energy between photosystem I and photosystem II in red algae. III. Quantum requirements of the induction of a state 2-state 1 transition. Biochim Biophys Acta. 1980 Aug 5;592(1):76–86. doi: 10.1016/0005-2728(80)90115-2. [DOI] [PubMed] [Google Scholar]
- Staehelin L. A., Arntzen C. J. Regulation of chloroplast membrane function: protein phosphorylation changes the spatial organization of membrane components. J Cell Biol. 1983 Nov;97(5 Pt 1):1327–1337. doi: 10.1083/jcb.97.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinback K. E., Bose S., Kyle D. J. Phosphorylation of the light-harvesting chlorophyll-protein regulates excitation energy distribution between photosystem II and photosystem I. Arch Biochem Biophys. 1982 Jun;216(1):356–361. doi: 10.1016/0003-9861(82)90221-1. [DOI] [PubMed] [Google Scholar]
- Thielen A. P., van Gorkom H. J. Quantum efficiency and antenna size of photosystems II alpha, II beta and I in tobacco chloroplasts. Biochim Biophys Acta. 1981 Mar 12;635(1):111–120. doi: 10.1016/0005-2728(81)90012-8. [DOI] [PubMed] [Google Scholar]
- Wollman F. A., Delepelaire P. Correlation between changes in light energy distribution and changes in thylakoid membrane polypeptide phosphorylation in Chlamydomonas reinhardtii. J Cell Biol. 1984 Jan;98(1):1–7. doi: 10.1083/jcb.98.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]


