Abstract
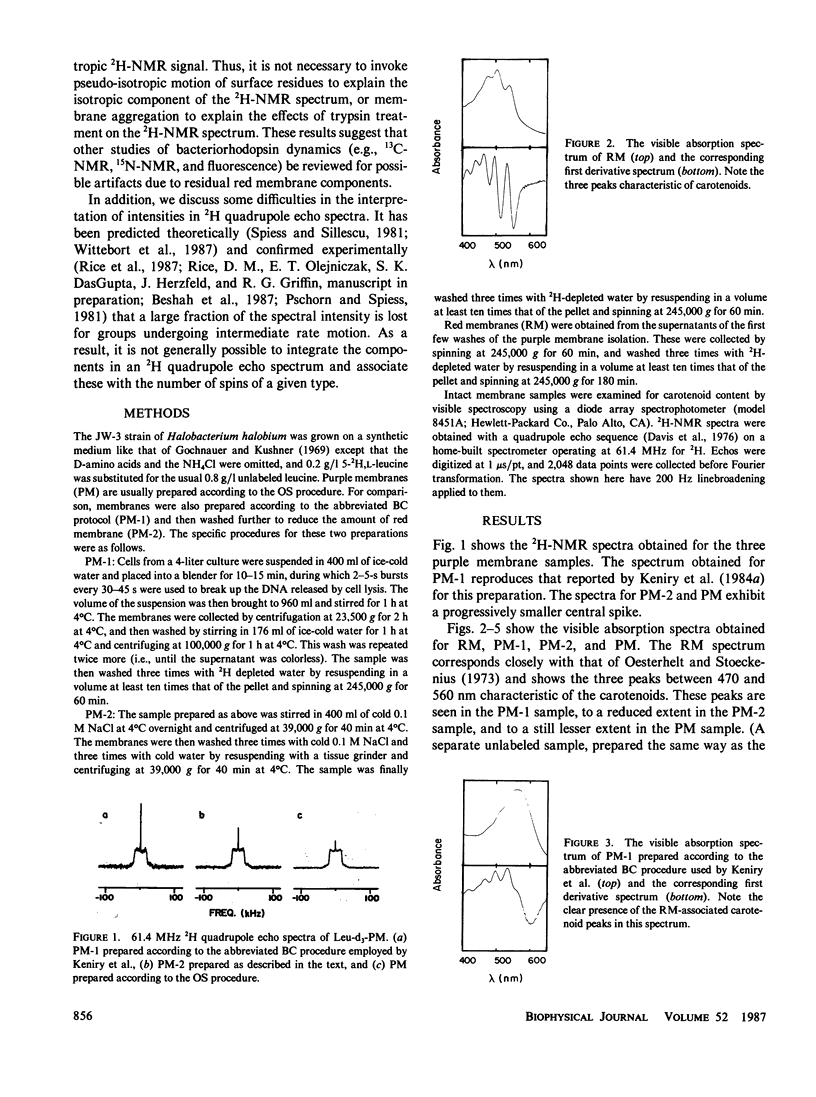
2H-nuclear magnetic resonance (NMR) has been used to study the dynamics of amino acid residues in bacteriorhodopsin with results that depend on the method of sample preparation. We show here that in [2H]-leucine-labeled samples the intensity of the isotropic signal varies according to the degree of residual contamination of the sample with red membrane. We conclude that few of the surface leucine residues of bacteriorhodopsin are moving isotropically on the 2H-NMR time scale.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becher B. M., Cassim J. Y. Improved isolation procedures for the purple membrane of Halobacterium halobium. Prep Biochem. 1975;5(2):161–178. doi: 10.1080/00327487508061568. [DOI] [PubMed] [Google Scholar]
- Gochnauer M. B., Kushner D. J. Growth and nutrition of extremely halophilic bacteria. Can J Microbiol. 1969 Oct;15(10):1157–1165. doi: 10.1139/m69-211. [DOI] [PubMed] [Google Scholar]
- Keniry M. A., Gutowsky H. S., Oldfield E. Surface dynamics of the integral membrane protein bacteriorhodopsin. 1984 Jan 26-Feb 1Nature. 307(5949):383–386. doi: 10.1038/307383a0. [DOI] [PubMed] [Google Scholar]
- Keniry M. A., Kintanar A., Smith R. L., Gutowsky H. S., Oldfield E. Nuclear magnetic resonance studies of amino acids and proteins. Deuterium nuclear magnetic resonance relaxation of deuteriomethyl-labeled amino acids in crystals and in Halobacterium halobium and Escherichia coli cell membranes. Biochemistry. 1984 Jan 17;23(2):288–298. doi: 10.1021/bi00297a018. [DOI] [PubMed] [Google Scholar]
- Kinsey R. A., Kintanar A., Oldfield E. Dynamics of amino acid side chains in membrane proteins by high field solid state deuterium nuclear magnetic resonance spectroscopy. Phenylalanine, tyrosine, and tryptophan. J Biol Chem. 1981 Sep 10;256(17):9028–9036. [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Isolation of the cell membrane of Halobacterium halobium and its fractionation into red and purple membrane. Methods Enzymol. 1974;31:667–678. doi: 10.1016/0076-6879(74)31072-5. [DOI] [PubMed] [Google Scholar]
- Smith R. L., Oldfield E. Dynamic structure of membranes by deuterium NMR. Science. 1984 Jul 20;225(4659):280–288. doi: 10.1126/science.6740310. [DOI] [PubMed] [Google Scholar]


