Abstract
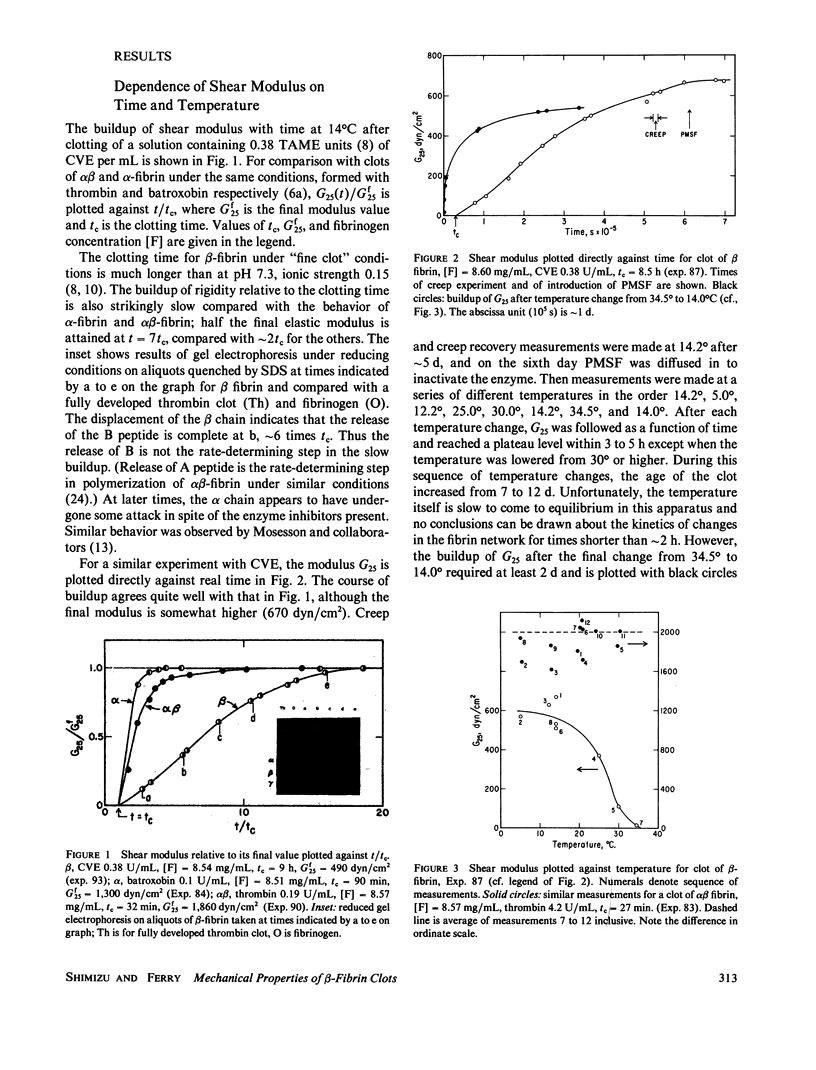
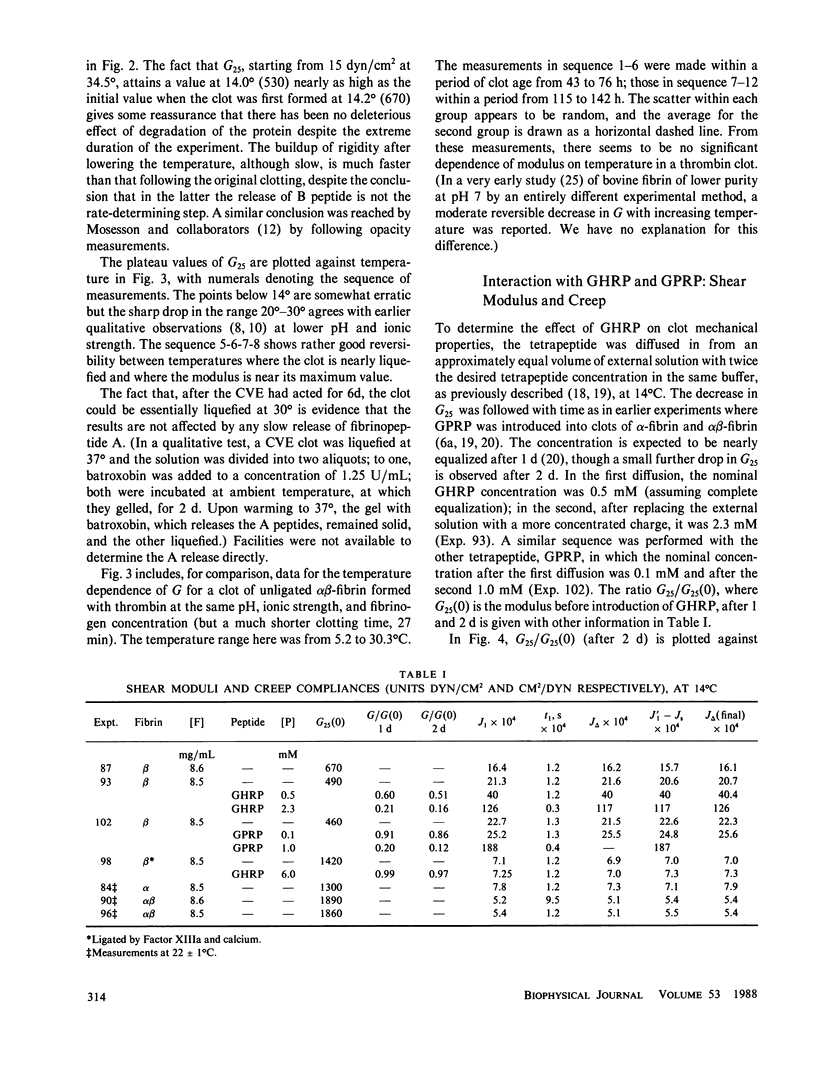
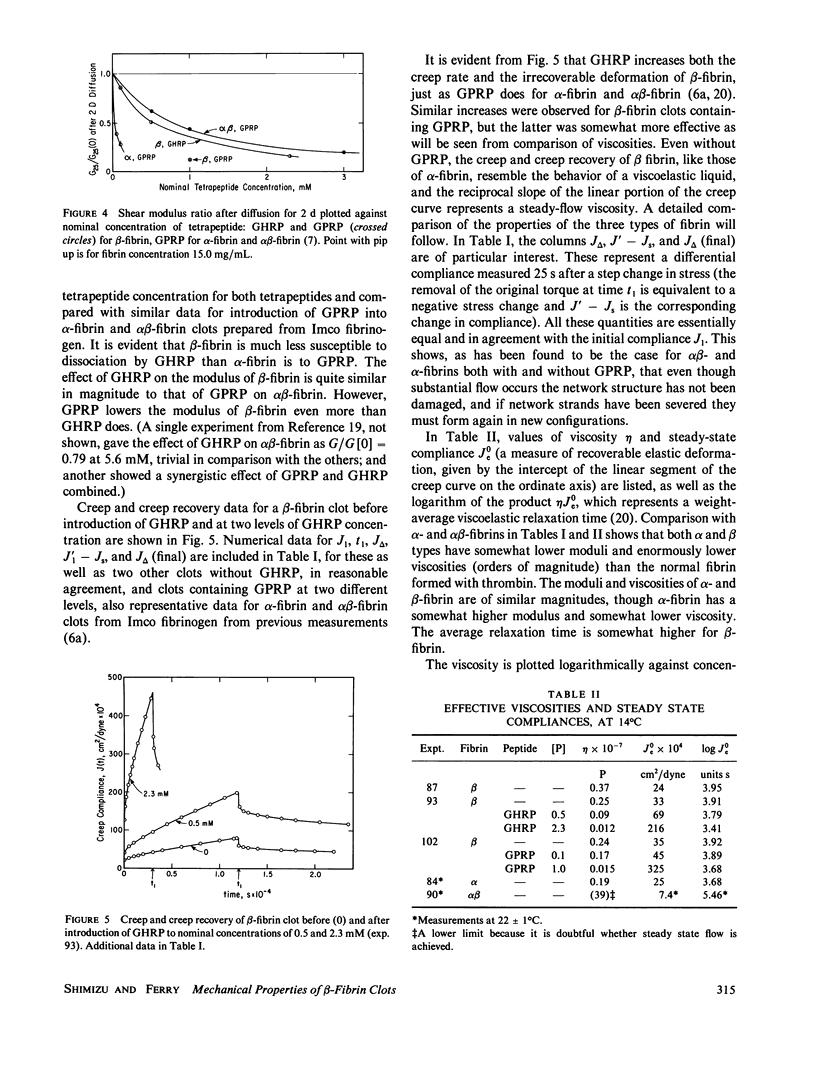
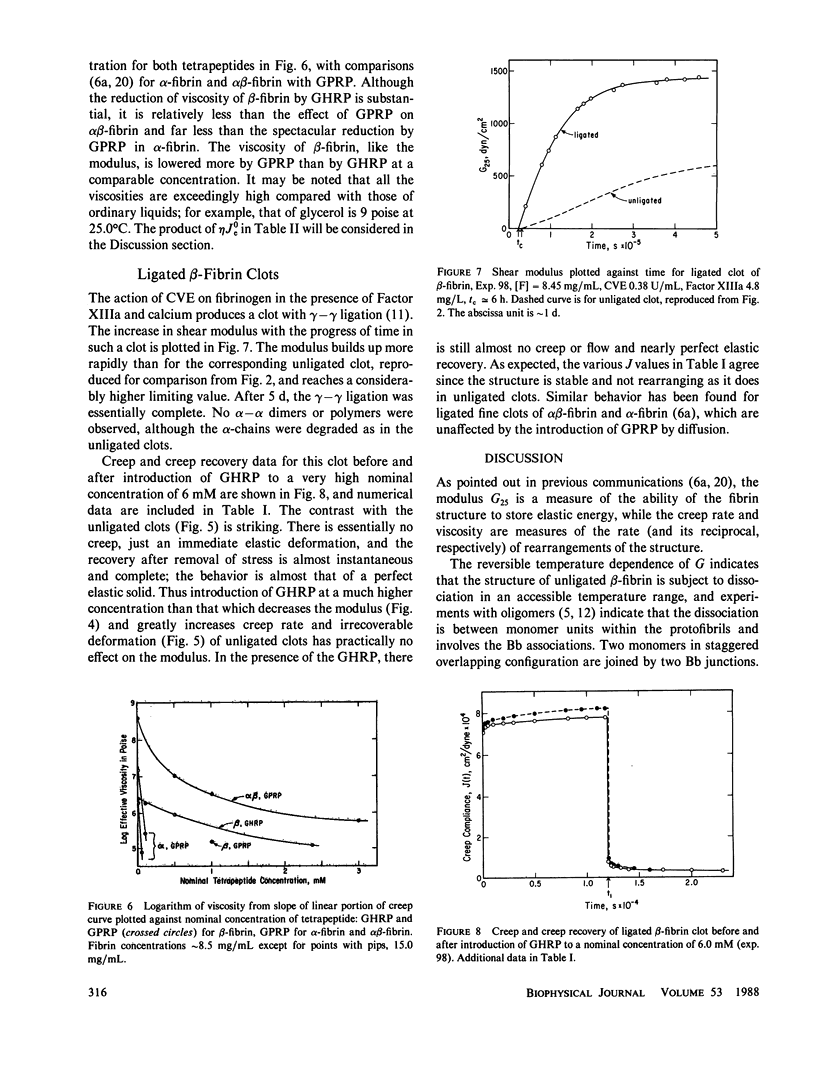
Clots of human beta-fibrin, in which only (or predominantly) the B fibrinopeptide is released, were formed at 14 degrees C by copperhead venom procoagulant enzyme (CVE or venzyme), at pH 8.5, ionic strength 0.45. The shear modulus of elasticity increased slowly and after several days attained a constant value, which was lower than those of alpha-fibrin or alpha beta-fibrin under the same conditions. Before studying the temperature dependence of elasticity, the CVE was then inhibited by introducing phenyl methyl sulfonyl chloride (PMSF) by diffusion. With increasing temperature, the modulus decreased progressively from 5 degrees C to nearly zero at 35 degrees and was essentially reversible with temperature change; recovery of elasticity after change from 34.5 degrees to 14 degrees required approximately 2 d but was considerably faster than the initial buildup of elasticity by CVE at 14 degrees. Creep and creep recovery measurements on unligated clots showed creep rates and irrecoverable deformation that were similar in magnitude to those of alpha-fibrin clots formed with batroxobin and much larger than those of alpha beta-fibrin clots formed with thrombin, under the same conditions. During creep and creep recovery, the differential modulus or compliance remained constant, showing that there was no permanent structural damage, and if network strands are severed in slow flow, they must rejoin in new configurations. Introduction (by diffusion) of the tetrapeptides Gly-His-Arg-Pro (GHRP) and Gly-Pro-Arg-Pro (GPRP), which resemble the B and A binding sites on the E domain of fibrin respectively, reduced the shear modulus and increased the creep rate of beta-fibrin clots to an extent similar to the effect of GPRP on alpha beta-fibrin, much more than that of GHRP on alpha beta-fibrin, but much less than that of GPRP on a-fibrin. A ligated beta-fibrin clot formed with Factor XIIIa (in which the activating thrombin had been neutralized by hirudin) showed essentially perfect elastic behavior, with no creep and with complete recovery after removal of stress, and was inert to GHRP.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bale M. D., Janmey P. A., Ferry J. D. Kinetics of formation of fibrin oligomers. II. Size distributions of ligated oligomers. Biopolymers. 1982 Nov;21(11):2265–2277. doi: 10.1002/bip.360211113. [DOI] [PubMed] [Google Scholar]
- Bale M. D., Müller M. F., Ferry J. D. Effects of fibrinogen-binding tetrapeptides on mechanical properties of fine fibrin clots. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1410–1413. doi: 10.1073/pnas.82.5.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale M. D., Müller M. F., Ferry J. D. Rheological studies of creep and creep recovery of unligated fibrin clots: comparison of clots prepared with thrombin and ancrod. Biopolymers. 1985 Mar;24(3):461–482. doi: 10.1002/bip.360240304. [DOI] [PubMed] [Google Scholar]
- Blombäck B., Hessel B., Hogg D., Therkildsen L. A two-step fibrinogen--fibrin transition in blood coagulation. Nature. 1978 Oct 12;275(5680):501–505. doi: 10.1038/275501a0. [DOI] [PubMed] [Google Scholar]
- Budzynski A. Z., Olexa S. A., Pandya B. V. Fibrin polymerization sites in fibrinogen and fibrin fragments. Ann N Y Acad Sci. 1983 Jun 27;408:301–314. doi: 10.1111/j.1749-6632.1983.tb23253.x. [DOI] [PubMed] [Google Scholar]
- Dardik B. N., Shainoff J. R. Crosslinking of monomeric fibrin by factor XIIIa. Thromb Haemost. 1979 Oct 31;42(3):864–872. [PubMed] [Google Scholar]
- Doolittle R. F. Fibrinogen and fibrin. Annu Rev Biochem. 1984;53:195–229. doi: 10.1146/annurev.bi.53.070184.001211. [DOI] [PubMed] [Google Scholar]
- FERRY J. D., MILLER M., SHULMAN S. The conversion of fibrinogen to fibrin. VII. Rigidity and stress relaxation of fibrin clots, eff. of calcium. Arch Biochem Biophys. 1951 Dec;34(2):424–436. doi: 10.1016/0003-9861(51)90021-5. [DOI] [PubMed] [Google Scholar]
- Furlan M., Rupp C., Beck E. A., Svendsen L. Effect of calcium and synthetic peptides on fibrin polymerization. Thromb Haemost. 1982 Apr 30;47(2):118–121. [PubMed] [Google Scholar]
- Furlan M., Seelich T., Beck E. A. Clottability and cross-linking reactivity of fibrin(ogen) following differential release of fibrinopeptides A and B. Thromb Haemost. 1976 Dec 31;36(3):582–592. [PubMed] [Google Scholar]
- Herzig R. H., Ratnoff O. D., Shainoff J. R. Studies on a procoagulant fraction of southern copperhead snake venom: the preferential release of fibrinopeptide B. J Lab Clin Med. 1970 Sep;76(3):451–465. [PubMed] [Google Scholar]
- Laudano A. P., Cottrell B. A., Doolittle R. F. Synthetic peptides modeled on fibrin polymerization sites. Ann N Y Acad Sci. 1983 Jun 27;408:315–329. doi: 10.1111/j.1749-6632.1983.tb23254.x. [DOI] [PubMed] [Google Scholar]
- Laudano A. P., Doolittle R. F. Studies on synthetic peptides that bind to fibrinogen and prevent fibrin polymerization. Structural requirements, number of binding sites, and species differences. Biochemistry. 1980 Mar 4;19(5):1013–1019. doi: 10.1021/bi00546a028. [DOI] [PubMed] [Google Scholar]
- Laudano A. P., Doolittle R. F. Synthetic peptide derivatives that bind to fibrinogen and prevent the polymerization of fibrin monomers. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3085–3089. doi: 10.1073/pnas.75.7.3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis S. D., Shields P. P., Shafer J. A. Characterization of the kinetic pathway for liberation of fibrinopeptides during assembly of fibrin. J Biol Chem. 1985 Aug 25;260(18):10192–10199. [PubMed] [Google Scholar]
- McKee P. A., Mattock P., Hill R. L. Subunit structure of human fibrinogen, soluble fibrin, and cross-linked insoluble fibrin. Proc Natl Acad Sci U S A. 1970 Jul;66(3):738–744. doi: 10.1073/pnas.66.3.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosesson M. W., DiOrio J. P., Müller M. F., Shainoff J. R., Siebenlist K. R., Amrani D. L., Homandberg G. A., Soria J., Soria C., Samama M. Studies on the ultrastructure of fibrin lacking fibrinopeptide B (beta-fibrin). Blood. 1987 Apr;69(4):1073–1081. [PubMed] [Google Scholar]
- Nelb G. W., Gerth C., Ferry J. D. Rheology of fibrin clots. III. Shear creep and creep recovery of fine ligated and coarse unligated closts. Biophys Chem. 1976 Sep;5(3):377–387. doi: 10.1016/0301-4622(76)80050-6. [DOI] [PubMed] [Google Scholar]
- Nelb G. W., Kamykowski G. W., Ferry J. D. Kinetics of ligation of fibrin oligomers. J Biol Chem. 1980 Jul 10;255(13):6398–6402. [PubMed] [Google Scholar]
- Nelb G. W., Kamykowski G. W., Ferry J. D. Rheology of fibrin clots. V. Shear modulus, creep, and creep recovery of fine unligated clots. Biophys Chem. 1981 Feb;13(1):15–23. doi: 10.1016/0301-4622(81)80020-8. [DOI] [PubMed] [Google Scholar]
- Olexa S. A., Budzynski A. Z. Evidence for four different polymerization sites involved in human fibrin formation. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1374–1378. doi: 10.1073/pnas.77.3.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindlauer G., Bale M. D., Ferry J. D. Interaction of fibrinogen-binding tetrapeptides with fibrin oligomers and fine fibrin clots. Biopolymers. 1986 Jul;25(7):1315–1336. doi: 10.1002/bip.360250711. [DOI] [PubMed] [Google Scholar]
- Shainoff J. R., Dardik B. N. Fibrinopeptide B and aggregation of fibrinogen. Science. 1979 Apr 13;204(4389):200–202. doi: 10.1126/science.155308. [DOI] [PubMed] [Google Scholar]
- Shainoff J. R., Dardik B. N. Fibrinopeptide B in fibrin assembly and metabolism: physiologic significance in delayed release of the peptide. Ann N Y Acad Sci. 1983 Jun 27;408:254–268. doi: 10.1111/j.1749-6632.1983.tb23249.x. [DOI] [PubMed] [Google Scholar]
- Stocker K., Fischer H., Meier J. Thrombin-like snake venom proteinases. Toxicon. 1982;20(1):265–273. doi: 10.1016/0041-0101(82)90225-2. [DOI] [PubMed] [Google Scholar]
- Weisel J. W. Fibrin assembly. Lateral aggregation and the role of the two pairs of fibrinopeptides. Biophys J. 1986 Dec;50(6):1079–1093. doi: 10.1016/S0006-3495(86)83552-4. [DOI] [PMC free article] [PubMed] [Google Scholar]



