Abstract
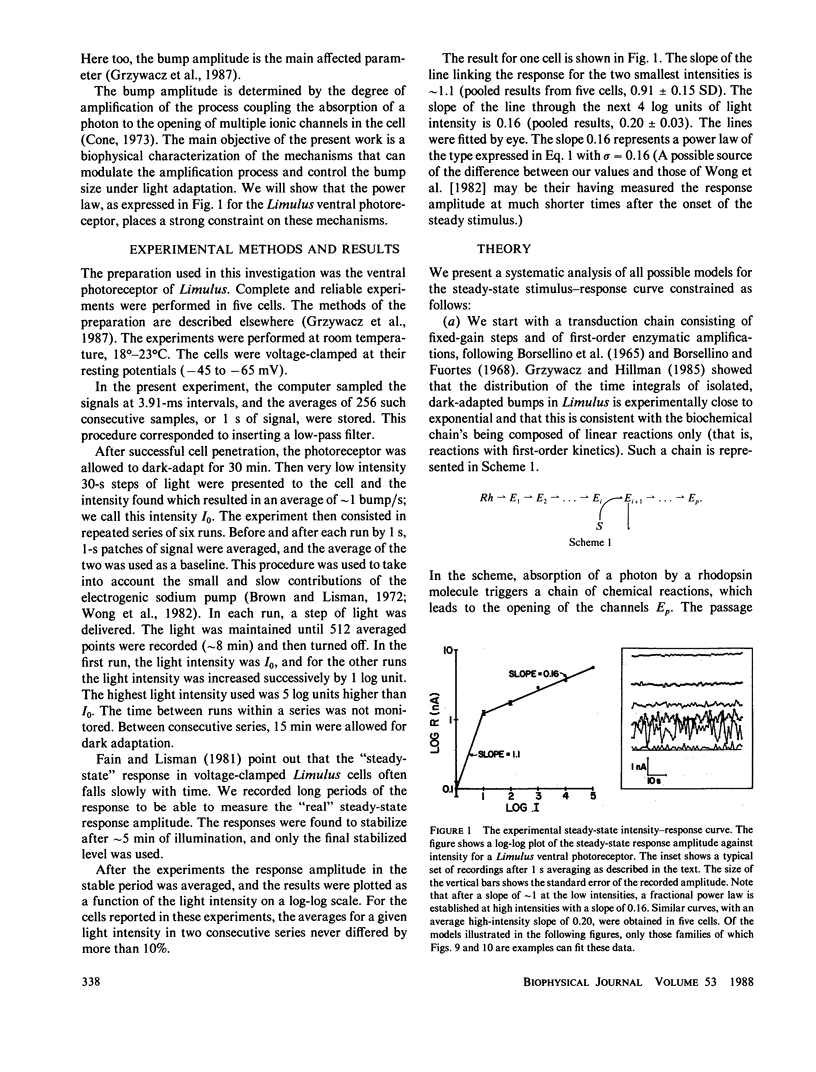
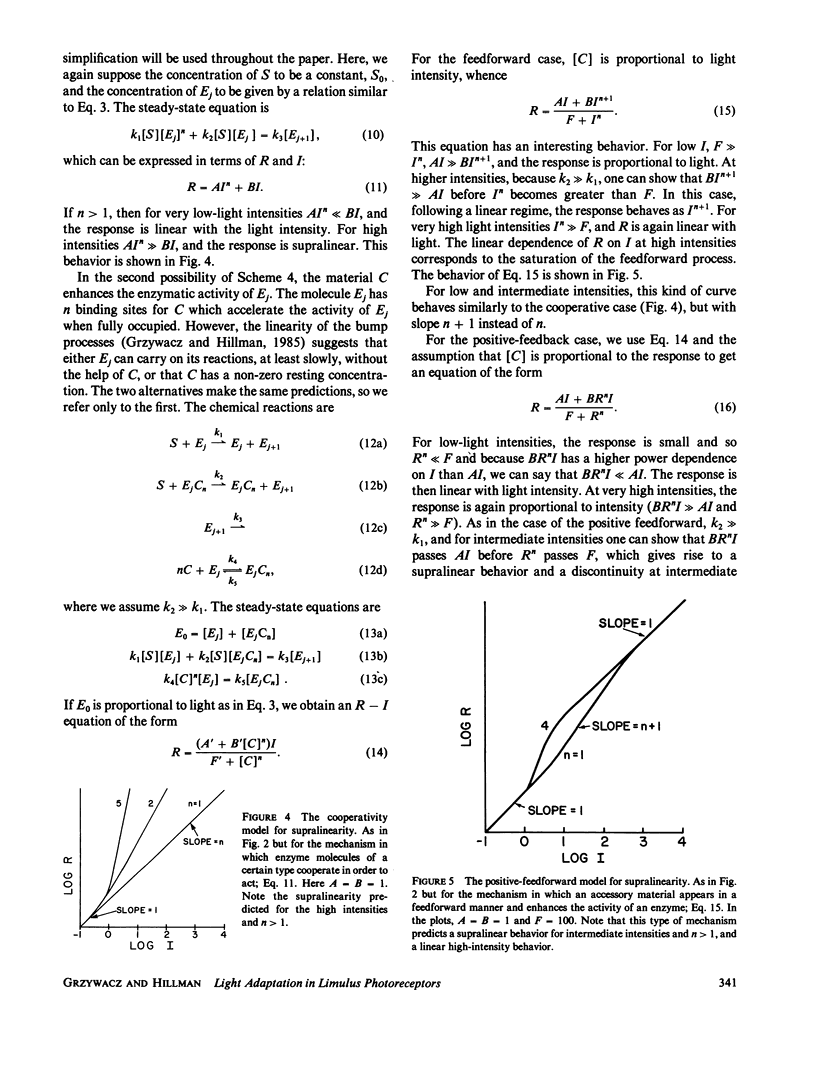
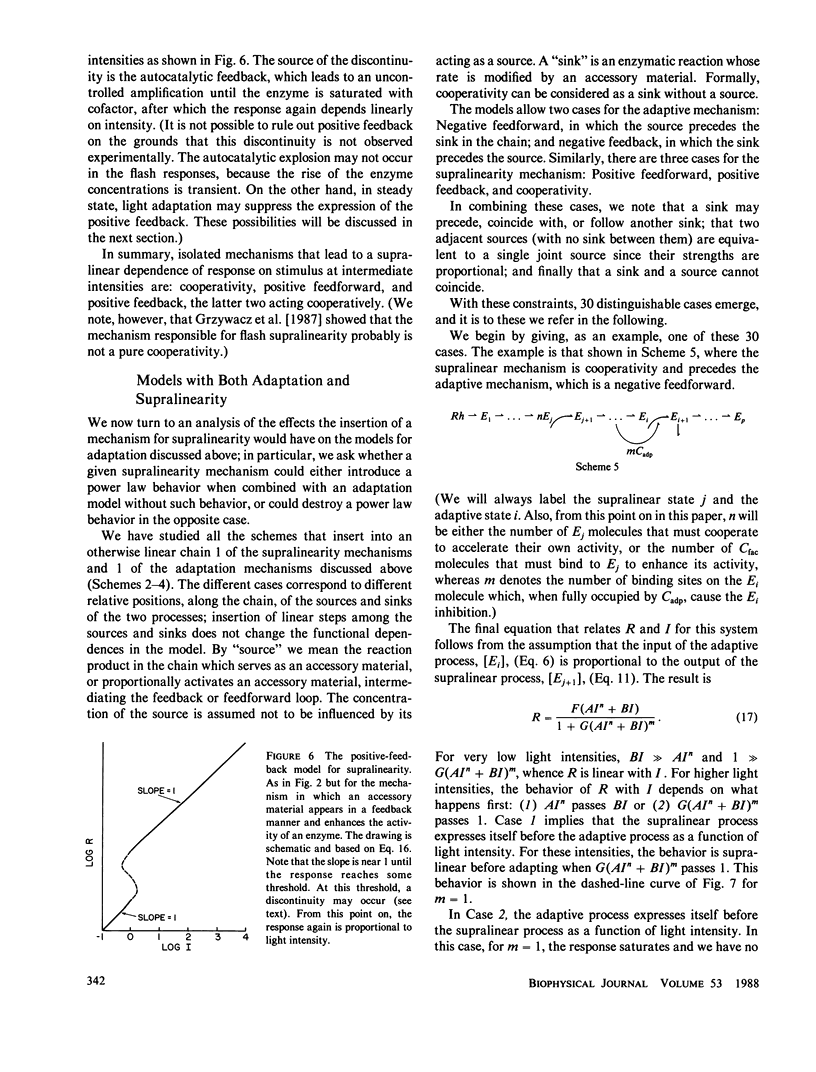
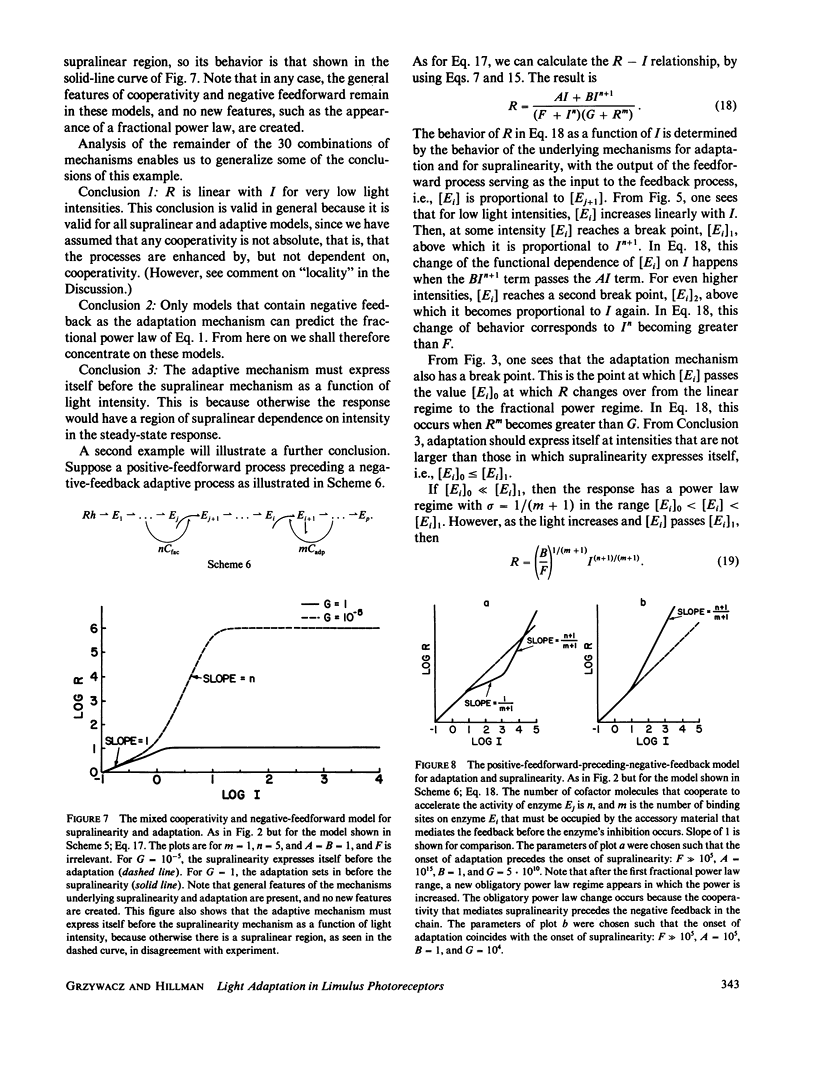
The steady-state stimulus-response curve of the Limulus ventral photoreceptor comprises a linear portion followed by a less-than-unity power law dependence, which is maintained over at least 4 decades of intensity. This progressive desensitization corresponds to light adaptation. For flash stimulation of dark-adapted cells, the stimulus-response curve again has an initial linear portion, but this is followed by a region of supralinearity before the curve saturates. In a previous article, we showed that the distribution of time integrals of the single-photon responses is consistent with a model of a single chain of first-order reactions. Starting with such a model, we have looked at relevant elementary nonlinear biochemical mechanisms to determine which of them can modulate the enzymatic amplifications of the chain in such a way as to lead to these behaviors. We assume that each of the two phenomena, adaptation and supralinearity, derives from a single mechanism that acts on a single enzymatic stage. We then conclude that the adaptation must be a cooperative negative feedback, in which an accessory material activated by a late stage of the transduction chain acts cooperatively to inhibit an earlier enzymatic amplification. In Limulus, the number of molecules that cooperate is between 3 and 5. We were not able to discard any of the mechanisms tested for the supralinearity, except to say that they must act at a stage of the chain later than that on which the adaptive material acts. If we assume the conclusions of a previous work which shows that the supralinearity mechanism is active during the steady state, we can also conclude that the supralinearity stage must precede the stage that is the source of the adaptive material.
Full text
PDF





Selected References
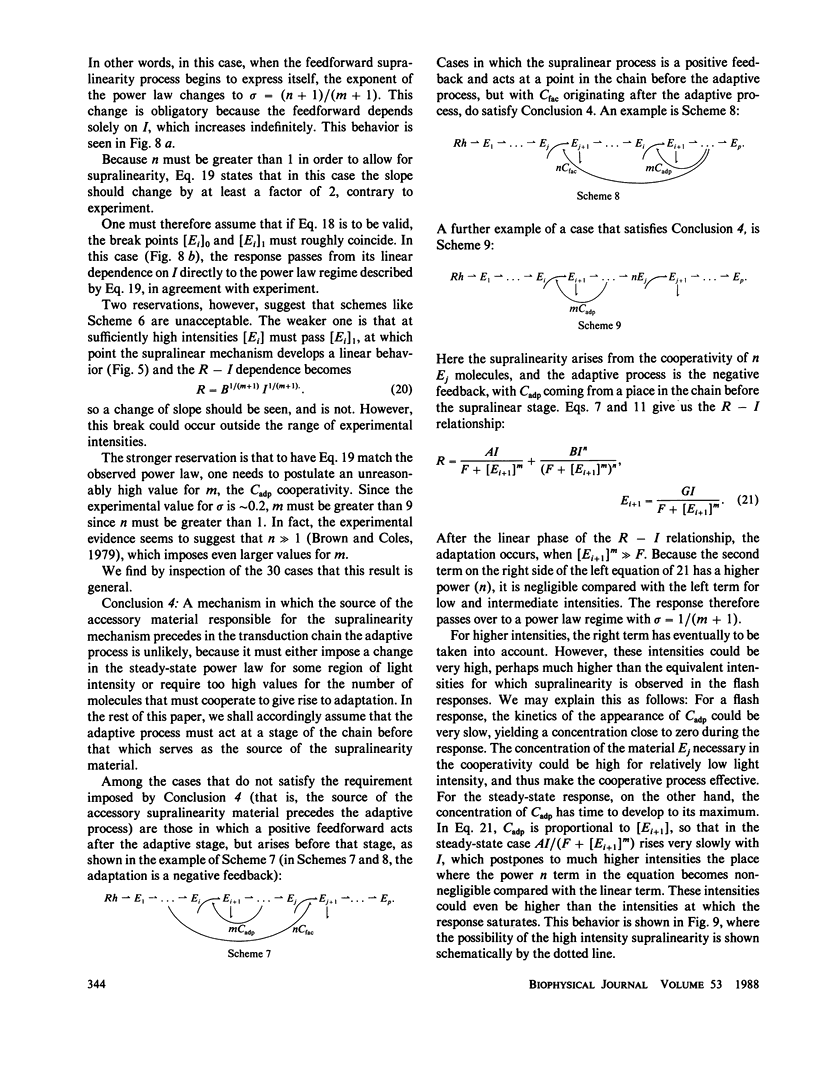
These references are in PubMed. This may not be the complete list of references from this article.
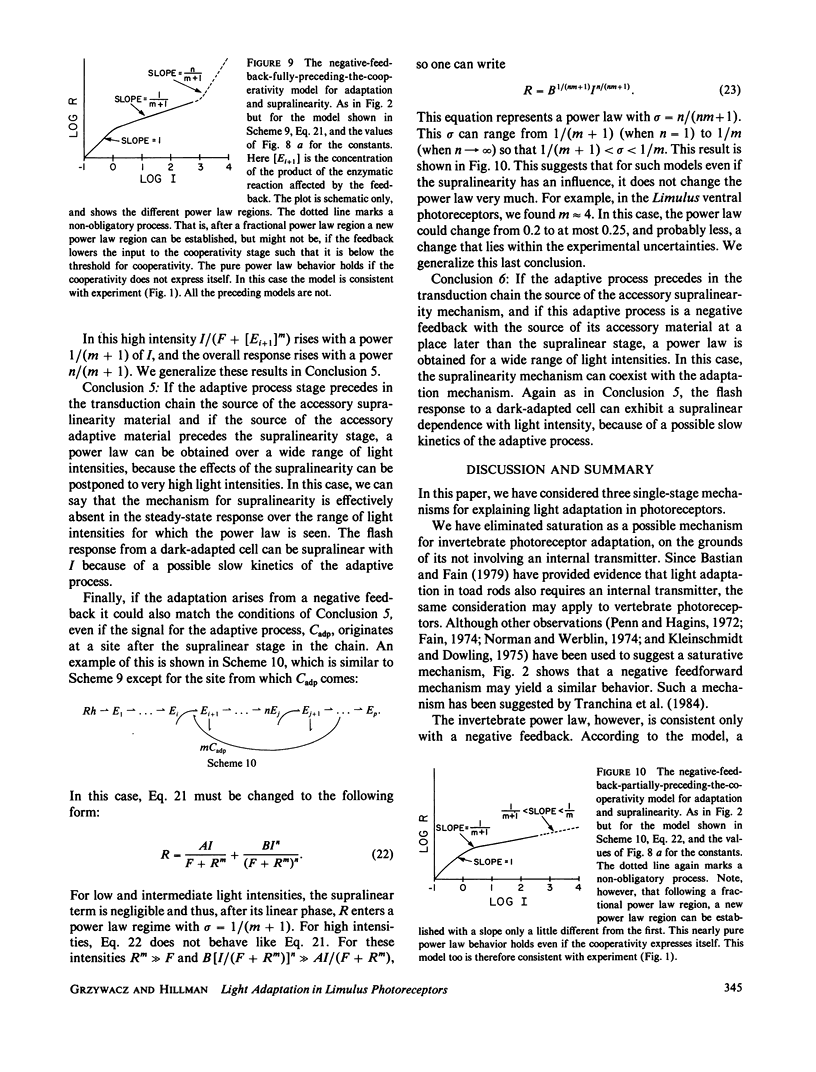
- Bader C. R., Baumann F., Bertrand D., Carreras J., Fuortes G. Diffuse and local effects of light adaptation in photoreceptors of the honey bee drone. Vision Res. 1982;22(2):311–317. doi: 10.1016/0042-6989(82)90131-6. [DOI] [PubMed] [Google Scholar]
- Bastian B. L., Fain G. L. Light adaptation in toad rods: requirement for an internal messenger which is not calcium. J Physiol. 1979 Dec;297(0):493–520. doi: 10.1113/jphysiol.1979.sp013053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsellino A., Fuortes M. G. Responses to single photons in virual cells of limulus. J Physiol. 1968 Jun;196(3):507–539. doi: 10.1113/jphysiol.1968.sp008521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsellino A., Fuortes M. G., Smith T. G. Visual responses in Limulus. Cold Spring Harb Symp Quant Biol. 1965;30:429–443. doi: 10.1101/sqb.1965.030.01.042. [DOI] [PubMed] [Google Scholar]
- Brown J. E., Coles J. A. Saturation of the response to light in Limulus ventral photoreceptor. J Physiol. 1979 Nov;296:373–392. doi: 10.1113/jphysiol.1979.sp013011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. E., Lisman J. E. An electrogenic sodium pump in Limulus ventral photoreceptor cells. J Gen Physiol. 1972 Jun;59(6):720–733. doi: 10.1085/jgp.59.6.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge F. A., Jr, Knight B. W., Toyoda J. Voltage noise in Limulus visual cells. Science. 1968 Apr 5;160(3823):88–90. doi: 10.1126/science.160.3823.88. [DOI] [PubMed] [Google Scholar]
- Fain G. L., Lisman J. E. Membrane conductances of photoreceptors. Prog Biophys Mol Biol. 1981;37(2):91–147. doi: 10.1016/0079-6107(82)90021-9. [DOI] [PubMed] [Google Scholar]
- Fein A., Charlton J. S. Local adaptation in the ventral photoreceptors of Limulus. J Gen Physiol. 1975 Dec;66(6):823–836. doi: 10.1085/jgp.66.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein A., Charlton J. S. Local membrane current in Limulus photoreceptors. Nature. 1975 Nov 20;258(5532):250–252. doi: 10.1038/258250a0. [DOI] [PubMed] [Google Scholar]
- Grzywacz N. M., Hillman P. Statistical test of linearity of photoreceptor transduction process: Limulus passes, others fail. Proc Natl Acad Sci U S A. 1985 Jan;82(1):232–235. doi: 10.1073/pnas.82.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman P. The biophysics of intermediate processes in photoreceptor transduction: 'silent' stages, non-localities, single-photon responses and models. Symp Soc Exp Biol. 1983;36:443–475. [PubMed] [Google Scholar]
- Klee C. B., Crouch T. H., Richman P. G. Calmodulin. Annu Rev Biochem. 1980;49:489–515. doi: 10.1146/annurev.bi.49.070180.002421. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt J., Dowling J. E. Intracellular recordings from gecko photoreceptors during light and dark adaptation. J Gen Physiol. 1975 Nov;66(5):617–648. doi: 10.1085/jgp.66.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer L., Widmann T. Quantitative model for the electric response of invertebrate and vertebrate photoreceptors. Biophys Struct Mech. 1977 Mar 2;2(4):333–336. doi: 10.1007/BF00537503. [DOI] [PubMed] [Google Scholar]
- Lisman J. E., Brown J. E. Light-induced changes of sensitivity in Limulus ventral photoreceptors. J Gen Physiol. 1975 Oct;66(4):473–488. doi: 10.1085/jgp.66.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J. E., Brown J. E. The effects of intracellular iontophoretic injection of calcium and sodium ions on the light response of Limulus ventral photoreceptors. J Gen Physiol. 1972 Jun;59(6):701–719. doi: 10.1085/jgp.59.6.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J. E. Effects of removing extracellular Ca2+ on excitation and adaptation in Limulus ventral photoreceptors. Biophys J. 1976 Nov;16(11):1331–1335. doi: 10.1016/S0006-3495(76)85777-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J. E., Strong J. A. The initiation of excitation and light adaptation in Limulus ventral photoreceptors. J Gen Physiol. 1979 Feb;73(2):219–243. doi: 10.1085/jgp.73.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Means A. R., Dedman J. R. Calmodulin in endocrine cells and its multiple roles in hormone action. Mol Cell Endocrinol. 1980 Sep;19(3):215–227. doi: 10.1016/0303-7207(80)90052-0. [DOI] [PubMed] [Google Scholar]
- Normann R. A., Werblin F. S. Control of retinal sensitivity. I. Light and dark adaptation of vertebrate rods and cones. J Gen Physiol. 1974 Jan;63(1):37–61. doi: 10.1085/jgp.63.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne R., Fein A. The initial response of Limulus ventral photoreceptors to bright flashes. Released calcium as a synergist to excitation. J Gen Physiol. 1986 Feb;87(2):243–269. doi: 10.1085/jgp.87.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn R. D., Hagins W. A. Kinetics of the photocurrent of retinal rods. Biophys J. 1972 Aug;12(8):1073–1094. doi: 10.1016/S0006-3495(72)86145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens S. S. Neural events and the psychophysical law. Science. 1970 Dec 4;170(3962):1043–1050. doi: 10.1126/science.170.3962.1043. [DOI] [PubMed] [Google Scholar]
- Tranchina D., Gordon J., Shapley R. M. Retinal light adaptation--evidence for a feedback mechanism. 1984 Jul 26-Aug 1Nature. 310(5975):314–316. doi: 10.1038/310314a0. [DOI] [PubMed] [Google Scholar]
- Wong F., Knight B. W., Dodge F. A. Adapting bump model for ventral photoreceptors of Limulus. J Gen Physiol. 1982 Jun;79(6):1089–1113. doi: 10.1085/jgp.79.6.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. F., Pak W. L. Light-induced voltage noise in the photoreceptor of Drosophila melanogaster. J Gen Physiol. 1978 Mar;71(3):249–268. doi: 10.1085/jgp.71.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]


