Abstract
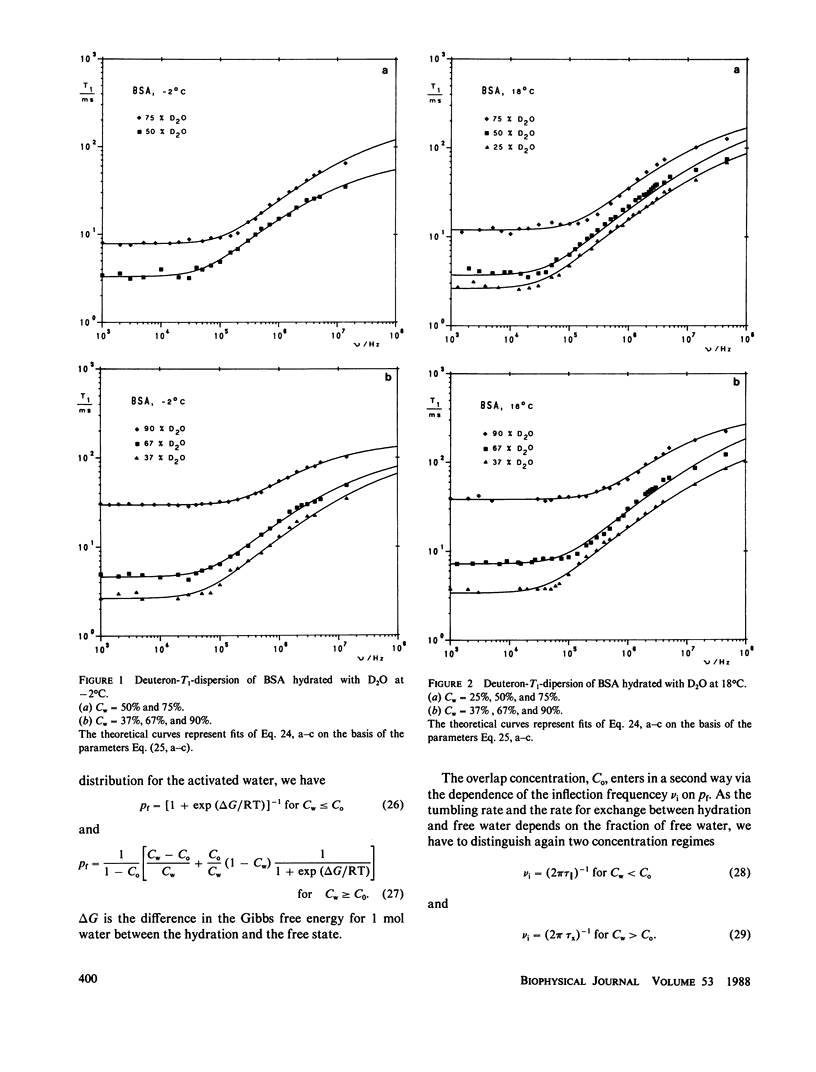
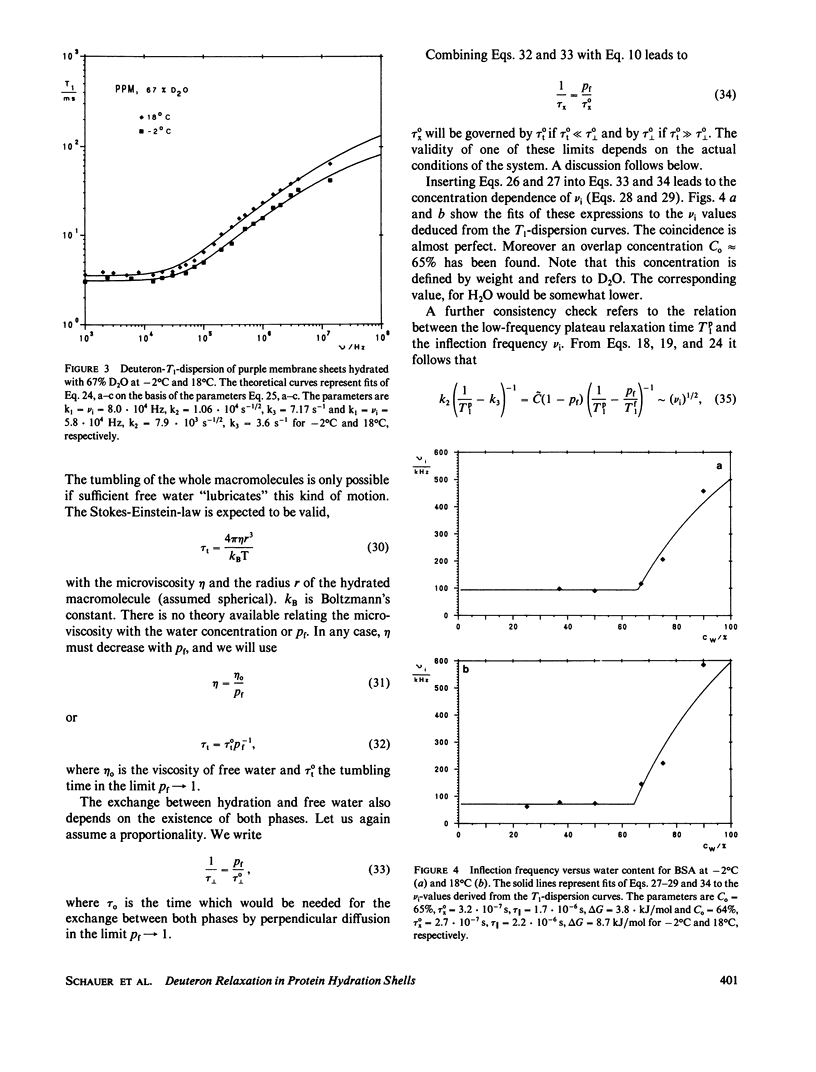
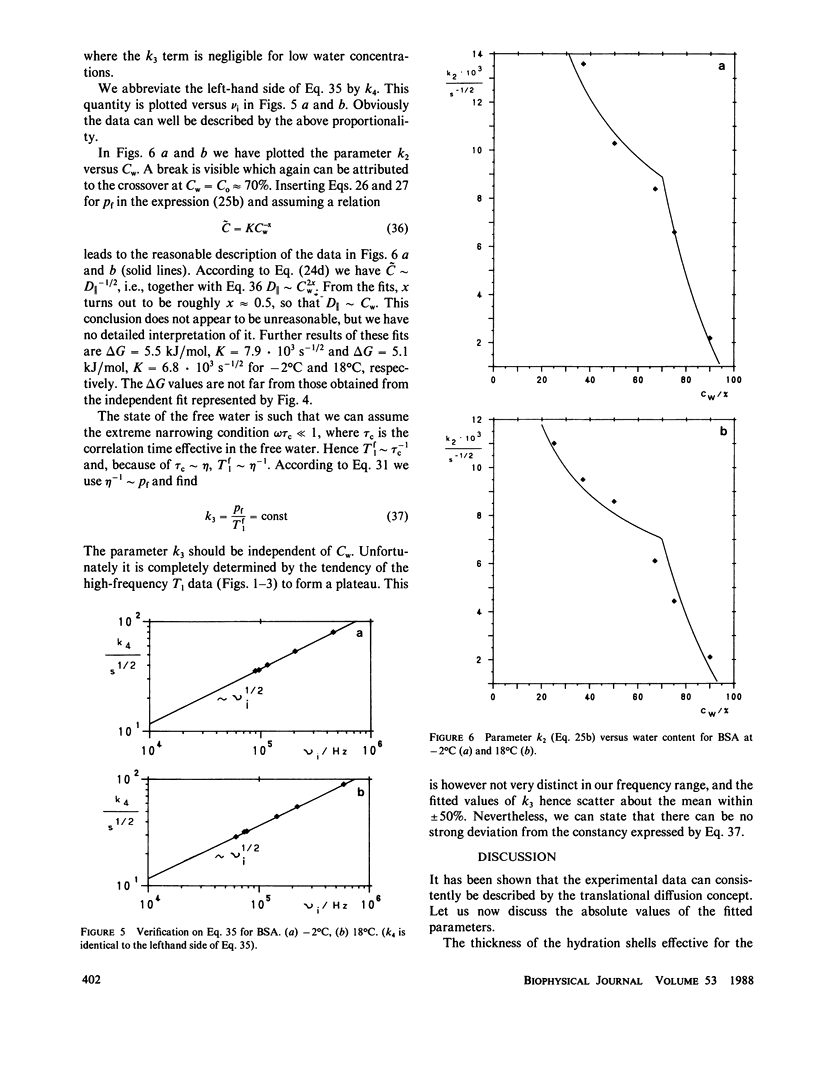
The deuterated hydration shells of bovine serum (BSA) albumin, and purple membrane sheets have been studied by the aid of deuteron field-cycling relaxation spectroscopy. The deuteron Larmor frequency range was 10(3) to 10(8) Hz. The temperature and the water content has been varied. The data distinguish translational diffusion on the protein surface from macromolecular tumbling or exchange with free water. A theory well describing all dependences has been developed on this basis. All parameters have successfully been tested concerning consistency with other sources of information. The concept is considered as a major relaxation scheme determining, apart from cross-relaxation effects, the water proton relaxation in tissue.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beall P. T., Hazlewood C. F., Rao P. N. Nuclear magnetic resonance patterns of intracellular water as a function of HeLa cell cycle. Science. 1976 May 28;192(4242):904–907. doi: 10.1126/science.1273575. [DOI] [PubMed] [Google Scholar]
- Blicharska B., Florkowski Z., Hennel J. W., Held G., Noack F. Investigation of protein hydration by proton spin relaxation time measurements. Biochim Biophys Acta. 1970 Jun 23;207(3):381–389. doi: 10.1016/s0005-2795(70)80001-0. [DOI] [PubMed] [Google Scholar]
- Damadian R., Zaner K., Hor D., DiMaio T. Human tumors detected by nuclear magnetic resonance. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1471–1473. doi: 10.1073/pnas.71.4.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doster W., Bachleitner A., Dunau R., Hiebl M., Lüscher E. Thermal properties of water in myoglobin crystals and solutions at subzero temperatures. Biophys J. 1986 Aug;50(2):213–219. doi: 10.1016/S0006-3495(86)83455-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escanye J. M., Canet D., Robert J. Frequency dependence of water proton longitudinal NMR relaxation times in mouse tissues around the freezing transition. Biochim Biophys Acta. 1983 Jun 2;762(3):445–451. doi: 10.1016/0167-4889(83)90010-1. [DOI] [PubMed] [Google Scholar]
- Fung B. M., Siegel M. M. The ordering and relaxation times of water adsorbed on collagen fibers. Biochim Biophys Acta. 1972 Aug 31;278(1):185–187. doi: 10.1016/0005-2795(72)90120-1. [DOI] [PubMed] [Google Scholar]
- Hallenga K., Koenig S. H. Protein rotational relaxation as studied by solvent 1H and 2H magnetic relaxation. Biochemistry. 1976 Sep 21;15(19):4255–4264. doi: 10.1021/bi00664a019. [DOI] [PubMed] [Google Scholar]
- Kasturi S. R., Chang D. C., Hazlewood C. F. Study of anisotropy in nuclear magnetic resonance relaxation times of water protons in skeletal muscle. Biophys J. 1980 Jun;30(3):369–381. doi: 10.1016/S0006-3495(80)85102-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmich R., Nusser W., Winter F. In vivo NMR field-cycling relaxation spectroscopy reveals 14N1H relaxation sinks in the backbones of proteins. Phys Med Biol. 1984 May;29(5):593–596. doi: 10.1088/0031-9155/29/5/011. [DOI] [PubMed] [Google Scholar]
- Koenig S. H., Hallenga K., Shporer M. Protein-water interaction studied by solvent 1H, 2H, and 17O magnetic relaxation. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2667–2671. doi: 10.1073/pnas.72.7.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig S. H., Schillinger W. E. Nuclear magnetic relaxation dispersion in protein solutions. I. Apotransferrin. J Biol Chem. 1969 Jun 25;244(12):3283–3289. [PubMed] [Google Scholar]
- Middendorf H. D., Randall J. T., Crespi H. L. Neutron spectroscopy of hydrogenous and biosynthetically deuterated proteins. Basic Life Sci. 1984;27:381–400. doi: 10.1007/978-1-4899-0375-4_23. [DOI] [PubMed] [Google Scholar]
- Spohn K. H., Kimmich R. Characterization of the mobility of various chemical groups in the purple membrane of Halobacterium halobium by 13C, 31P and 2H solid state NMR. Biochem Biophys Res Commun. 1983 Jul 29;114(2):713–720. doi: 10.1016/0006-291x(83)90839-2. [DOI] [PubMed] [Google Scholar]


