Abstract
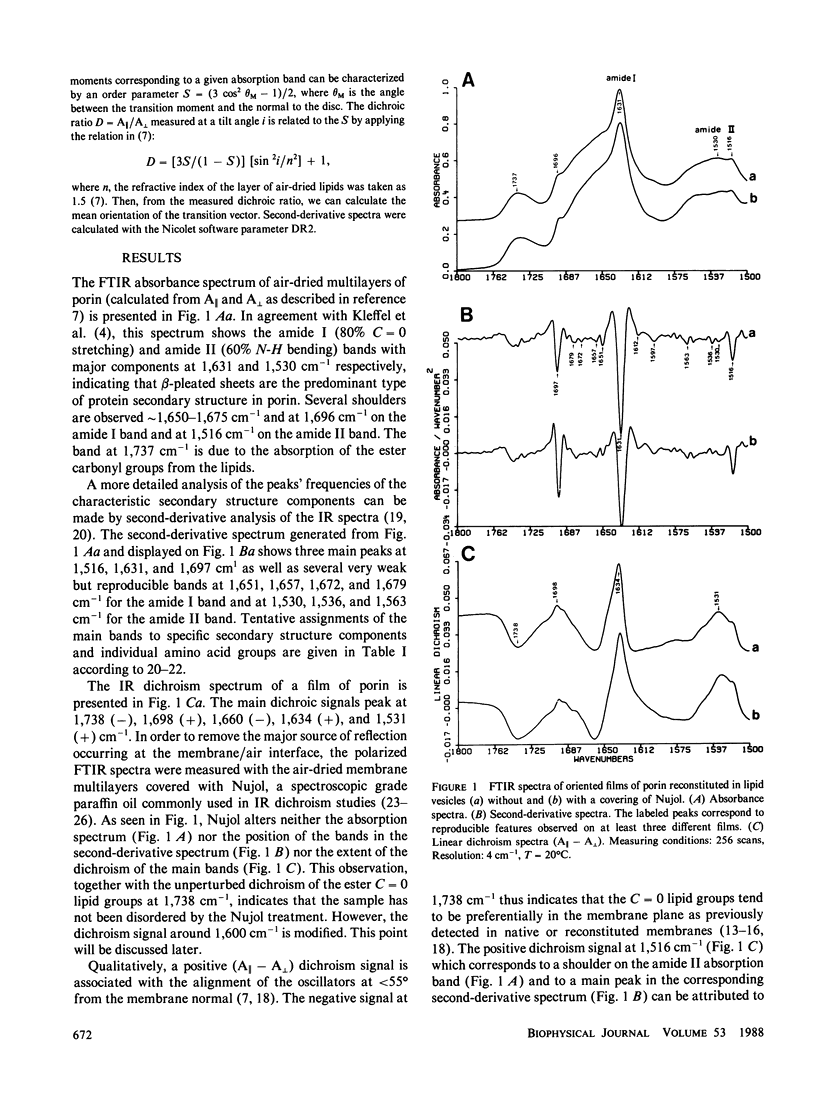
The orientation of the protein secondary structures in porin is investigated by Fourier transform infrared (FTIR) linear dichroism of oriented multilayers of porin reconstituted in lipid vesicles. The FTIR absorbance spectrum shows the amide I band at 1,631 cm-1 and several shoulders around 1,675 cm-1 and at 1,696 cm-1 indicative of antiparallel beta-sheets. The amide II is centered around 1,530 cm-1. The main dichroic signals peak at 1,738, 1,698, 1,660, 1,634, and 1,531 cm-1. The small magnitude of the 1,634 cm-1 and 1,531 cm-1 positive dichroism bands demonstrates that the transition moments of the amide I and amide II vibrations are on the average tilted at 47 degrees +/- 3 degrees from the membrane normal. This indicates that the plane of the beta-sheets is approximately perpendicular to the bilayer. From these IR dichroism results and previously reported diffuse x-ray data which revealed that a substantial number of beta-strands are nearly perpendicular to the membrane, a model for the packing of beta-strands in porin is proposed which satisfies both IR and x-ray requirements. In this model, the porin monomer consists of at least two beta-sheet domains, both with their plane perpendicular to the membrane. One sheet has its strands direction lying nearly parallel to the membrane normal while the other sheet has its strands inclined at a small angle away from the membrane plane.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bandekar J., Krimm S. Vibrational analysis of peptides, polypeptides, and proteins: Characteristic amide bands of beta-turns. Proc Natl Acad Sci U S A. 1979 Feb;76(2):774–777. doi: 10.1073/pnas.76.2.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzi M. D., Woody R. W. Oriented secondary structure in integral membrane proteins. I. Circular dichroism and infrared spectroscopy of cytochrome oxidase in multilamellar films. Biophys J. 1985 Dec;48(6):957–966. doi: 10.1016/S0006-3495(85)83859-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byler D. M., Susi H. Examination of the secondary structure of proteins by deconvolved FTIR spectra. Biopolymers. 1986 Mar;25(3):469–487. doi: 10.1002/bip.360250307. [DOI] [PubMed] [Google Scholar]
- Chothia C., Janin J. Orthogonal packing of beta-pleated sheets in proteins. Biochemistry. 1982 Aug 17;21(17):3955–3965. doi: 10.1021/bi00260a009. [DOI] [PubMed] [Google Scholar]
- Dorset D. L., Engel A., Häner M., Massalski A., Rosenbusch J. P. Two-dimensional crystal packing of matrix porin. A channel forming protein in Escherichia coli outer membranes. J Mol Biol. 1983 Apr 25;165(4):701–710. doi: 10.1016/s0022-2836(83)80275-7. [DOI] [PubMed] [Google Scholar]
- Engel A., Massalski A., Schindler H., Dorset D. L., Rosenbusch J. P. Porin channel triplets merge into single outlets in Escherichia coli outer membranes. Nature. 1985 Oct 17;317(6038):643–645. doi: 10.1038/317643a0. [DOI] [PubMed] [Google Scholar]
- Garavito R. M., Jenkins J., Jansonius J. N., Karlsson R., Rosenbusch J. P. X-ray diffraction analysis of matrix porin, an integral membrane protein from Escherichia coli outer membranes. J Mol Biol. 1983 Feb 25;164(2):313–327. doi: 10.1016/0022-2836(83)90079-7. [DOI] [PubMed] [Google Scholar]
- Kennedy S. J. Structures of membrane proteins. J Membr Biol. 1978 Sep 19;42(3):265–279. doi: 10.1007/BF01870362. [DOI] [PubMed] [Google Scholar]
- Kleffel B., Garavito R. M., Baumeister W., Rosenbusch J. P. Secondary structure of a channel-forming protein: porin from E. coli outer membranes. EMBO J. 1985 Jun;4(6):1589–1592. doi: 10.1002/j.1460-2075.1985.tb03821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel-Villaz M., Saibil H. R., Chabre M. Orientation of rhodopsin alpha-helices in in retinal rod outer segment membranes studied by infrared linear dichroism. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4405–4408. doi: 10.1073/pnas.76.9.4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabedryk E., Bardin A. M., Breton J. Further characterization of protein secondary structures in purple membrane by circular dichroism and polarized infrared spectroscopies. Biophys J. 1985 Dec;48(6):873–876. doi: 10.1016/S0006-3495(85)83848-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabedryk E., Breton J. Orientation of intrinsic proteins in photosynthetic membranes. Polarized infrared spectroscopy of chloroplasts and chromatophores. Biochim Biophys Acta. 1981 May 13;635(3):515–524. doi: 10.1016/0005-2728(81)90110-9. [DOI] [PubMed] [Google Scholar]
- Nabedryk E., Gingold M. P., Breton J. Orientation of gramicidin A transmembrane channel. Infrared dichroism study of gramicidin in vesicles. Biophys J. 1982 Jun;38(3):243–249. doi: 10.1016/S0006-3495(82)84555-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul C., Rosenbusch J. P. Folding patterns of porin and bacteriorhodopsin. EMBO J. 1985 Jun;4(6):1593–1597. doi: 10.1002/j.1460-2075.1985.tb03822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbusch J. P. Characterization of the major envelope protein from Escherichia coli. Regular arrangement on the peptidoglycan and unusual dodecyl sulfate binding. J Biol Chem. 1974 Dec 25;249(24):8019–8029. [PubMed] [Google Scholar]
- Rothschild K. J., Clark N. A. Polarized infrared spectroscopy of oriented purple membrane. Biophys J. 1979 Mar;25(3):473–487. doi: 10.1016/S0006-3495(79)85317-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothschild K. J., Sanches R., Hsiao T. L., Clark N. A. A spectroscopic study of rhodopsin alpha-helix orientation. Biophys J. 1980 Jul;31(1):53–64. doi: 10.1016/S0006-3495(80)85040-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler H., Rosenbusch J. P. Matrix protein in planar membranes: clusters of channels in a native environment and their functional reassembly. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2302–2306. doi: 10.1073/pnas.78.4.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susi H., Byler D. M. Protein structure by Fourier transform infrared spectroscopy: second derivative spectra. Biochem Biophys Res Commun. 1983 Aug 30;115(1):391–397. doi: 10.1016/0006-291x(83)91016-1. [DOI] [PubMed] [Google Scholar]
- Tiede D. M. Incorporation of membrane proteins into interfacial films: model membranes for electrical and structural characterization. Biochim Biophys Acta. 1985 Dec;811(4):357–379. doi: 10.1016/0304-4173(85)90007-2. [DOI] [PubMed] [Google Scholar]
- Vogel H., Jähnig F. Models for the structure of outer-membrane proteins of Escherichia coli derived from raman spectroscopy and prediction methods. J Mol Biol. 1986 Jul 20;190(2):191–199. doi: 10.1016/0022-2836(86)90292-5. [DOI] [PubMed] [Google Scholar]


