Abstract
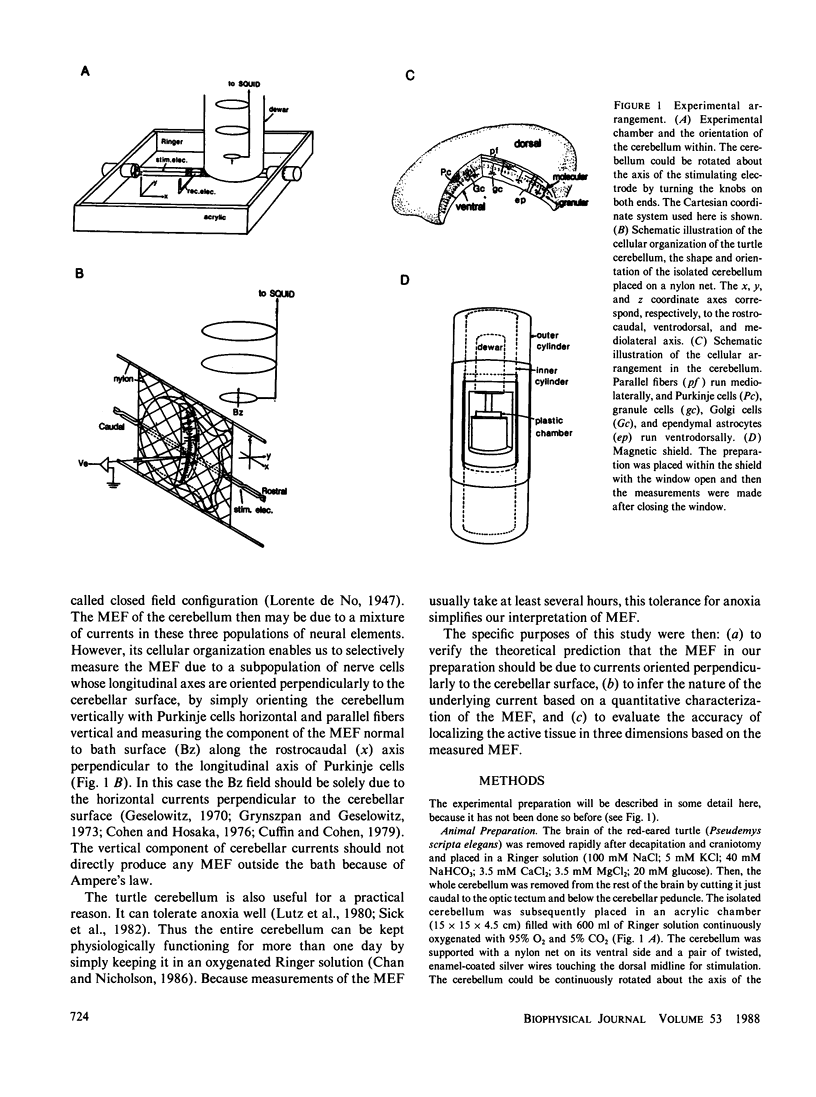
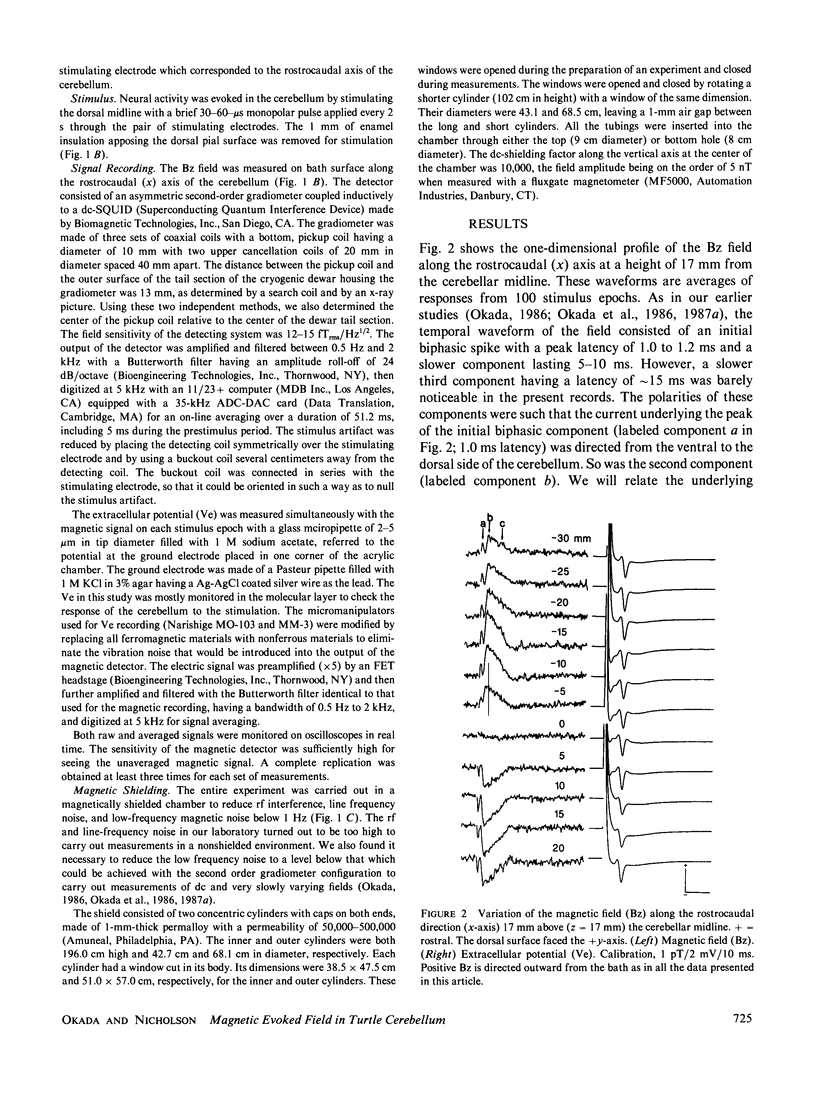
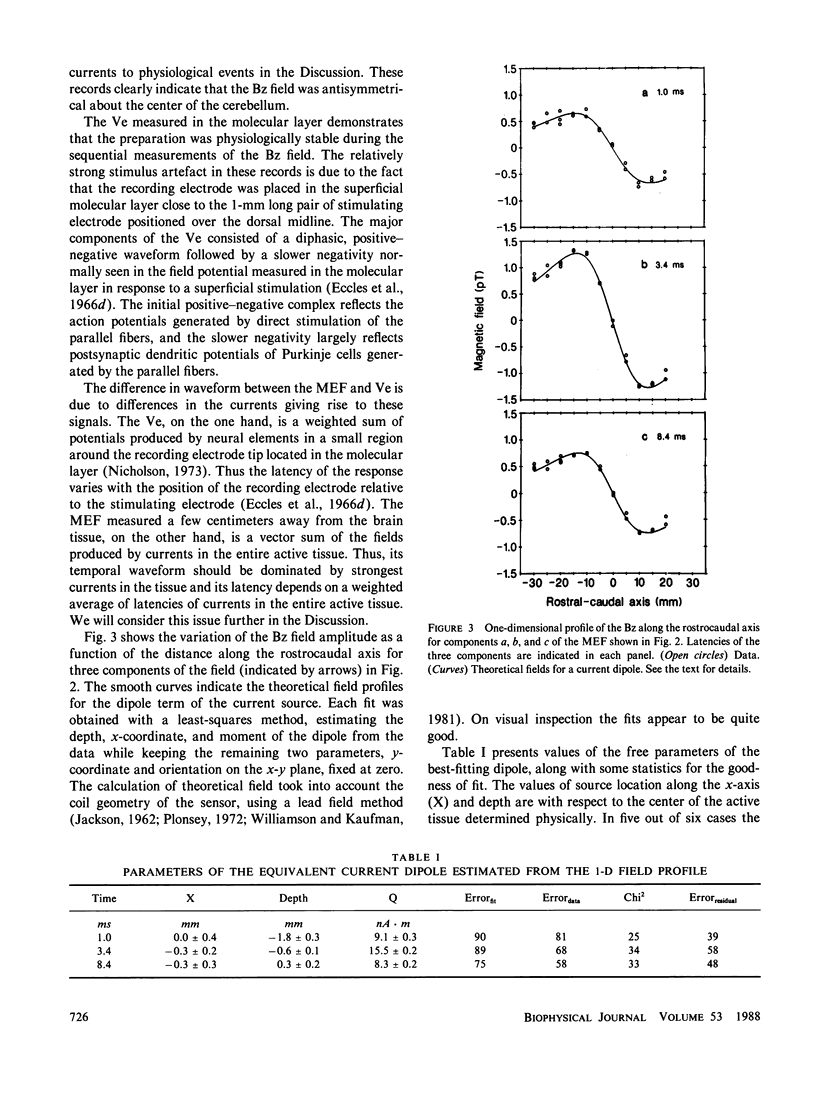
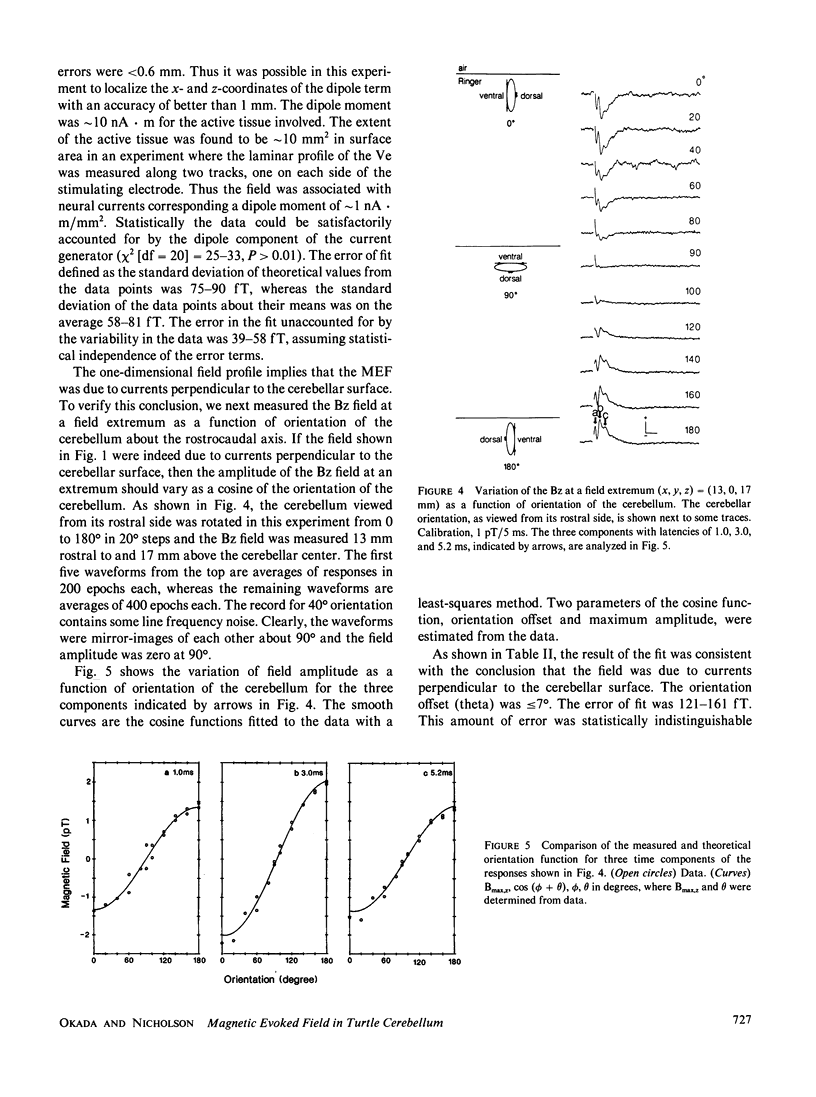
The neural basis of magnetic evoked fields of the brain was studied with an isolated turtle cerebellum as a model preparation. The turtle cerebellum is a nearly flat tissue with neural processes arranged along three orthogonal axes of symmetry. According to theoretical results, this geometry should enable us to selectively measure the magnetic field due to a subpopulation of nerve cells whose longitudinal axes are perpendicular to the cerebellar surface, by simply placing the cerebellum vertically in the bath so that these cells are horizontal and by measuring the field along the rostrocaudal axis perpendicular to the longitudinal axis of these nerve cells. To elicit neural activity in these cells the dorsal midline was electrically stimulated with a bipolar electrode. Consistent with our expectations, the one-dimensional profile of the field normal to bath surface (Bz) was antisymmetrical along the rostrocaudal axis, implying that the underlying currents were transcortical. Also, the Bz field at a field extremum varied as a cosine of the orientation of the cerebellum when it was rotated about its rostrocaudal axis with the amplitude being zero when the cerebellum was horizontal. The Bz field was dipolar as judged by statistically excellent fits of the dipolar field to the one-dimensional field profile and to the distance function relating the field magnitude at an extremum to measuring distance. This was the case even for the initial component thought to be due to antidromic action currents invading the soma and dendrites of Purkinje cells. We also showed that the dipolar term of the source could be localized within 1 mm of the actual source location in most cases.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bantli H. Analysis of difference between potentials evoked by climbing fibers in cerebellum of cat and turtle. J Neurophysiol. 1974 Jul;37(4):573–593. doi: 10.1152/jn.1974.37.4.573. [DOI] [PubMed] [Google Scholar]
- Barth D. S., Sutherling W., Beatty J. Fast and slow magnetic phenomena in focal epileptic seizures. Science. 1984 Nov 16;226(4676):855–857. doi: 10.1126/science.6436979. [DOI] [PubMed] [Google Scholar]
- Barth D. S., Sutherling W., Beatty J. Intracellular currents of interictal penicillin spikes: evidence from neuromagnetic mapping. Brain Res. 1986 Mar 12;368(1):36–48. doi: 10.1016/0006-8993(86)91040-1. [DOI] [PubMed] [Google Scholar]
- Chan C. Y., Nicholson C. Modulation by applied electric fields of Purkinje and stellate cell activity in the isolated turtle cerebellum. J Physiol. 1986 Feb;371:89–114. doi: 10.1113/jphysiol.1986.sp015963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen D., Hosaka H. Part II: magnetic field produced by a current dipole. J Electrocardiol. 1976;9(4):409–417. doi: 10.1016/s0022-0736(76)80041-6. [DOI] [PubMed] [Google Scholar]
- Cuffin B. N., Cohen D. Comparison of the magnetoencephalogram and electroencephalogram. Electroencephalogr Clin Neurophysiol. 1979 Aug;47(2):132–146. doi: 10.1016/0013-4694(79)90215-3. [DOI] [PubMed] [Google Scholar]
- Eccles J. C., Llinás R., Sasaki K. Intracellularly recorded responses of the cerebellar Purkinje cells. Exp Brain Res. 1966;1(2):161–183. doi: 10.1007/BF00236869. [DOI] [PubMed] [Google Scholar]
- Eccles J. C., Llinás R., Sasaki K. The action of antidromic impulses on the cerebellar Purkinje cells. J Physiol. 1966 Jan;182(2):316–345. doi: 10.1113/jphysiol.1966.sp007826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles J. C., Llinás R., Sasaki K. The mossy fibre-granule cell relay of the cerebellum and its inhibitory control by Golgi cells. Exp Brain Res. 1966;1(1):82–101. doi: 10.1007/BF00235211. [DOI] [PubMed] [Google Scholar]
- Eccles J. C., Llinás R., Sasaki K., Voorhoeve P. E. Interaction experiments on the responses evoked in Purkinje cells by climbing fibres. J Physiol. 1966 Jan;182(2):297–315. doi: 10.1113/jphysiol.1966.sp007825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynszpan F., Geselowitz D. B. Model studies of the magnetocardiogram. Biophys J. 1973 Sep;13(9):911–925. doi: 10.1016/S0006-3495(73)86034-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Künzle H. Climbing fiber projection to the turtle cerebellum: longitudinally oriented terminal zones within the basal third of the molecular layer. Neuroscience. 1985 Jan;14(1):159–168. doi: 10.1016/0306-4522(85)90171-x. [DOI] [PubMed] [Google Scholar]
- Llinas R., Bloedel J. R., Hillman D. E. Functional characterization of neuronal circuitry of frog cerebellar cortex. J Neurophysiol. 1969 Nov;32(6):847–870. doi: 10.1152/jn.1969.32.6.847. [DOI] [PubMed] [Google Scholar]
- Lutz P. L., LaManna J. C., Adams M. R., Rosenthal M. Cerebral resistance to anoxia in the marine turtle. Respir Physiol. 1980 Sep;41(3):241–251. doi: 10.1016/0034-5687(80)90074-2. [DOI] [PubMed] [Google Scholar]
- Nicholson C. Theoretical analysis of field potentials in anisotropic ensembles of neuronal elements. IEEE Trans Biomed Eng. 1973 Jul;20(4):278–288. doi: 10.1109/TBME.1973.324192. [DOI] [PubMed] [Google Scholar]
- Okada Y. C., Lauritzen M., Nicholson C. Magnetic field associated with neural activities in an isolated cerebellum. Brain Res. 1987 May 26;412(1):151–155. doi: 10.1016/0006-8993(87)91451-x. [DOI] [PubMed] [Google Scholar]
- Okada Y., Lauritzen M., Nicholson C. MEG source models and physiology. Phys Med Biol. 1987 Jan;32(1):43–51. doi: 10.1088/0031-9155/32/1/007. [DOI] [PubMed] [Google Scholar]
- Plonsey R. Capability and limitations of electrocardiography and magnetocardiography. IEEE Trans Biomed Eng. 1972 May;19(3):239–244. doi: 10.1109/TBME.1972.324123. [DOI] [PubMed] [Google Scholar]
- Sick T. J., Rosenthal M., LaManna J. C., Lutz P. L. Brain potassium ion homeostasis, anoxia, and metabolic inhibition in turtles and rats. Am J Physiol. 1982 Sep;243(3):R281–R288. doi: 10.1152/ajpregu.1982.243.3.R281. [DOI] [PubMed] [Google Scholar]


