Abstract
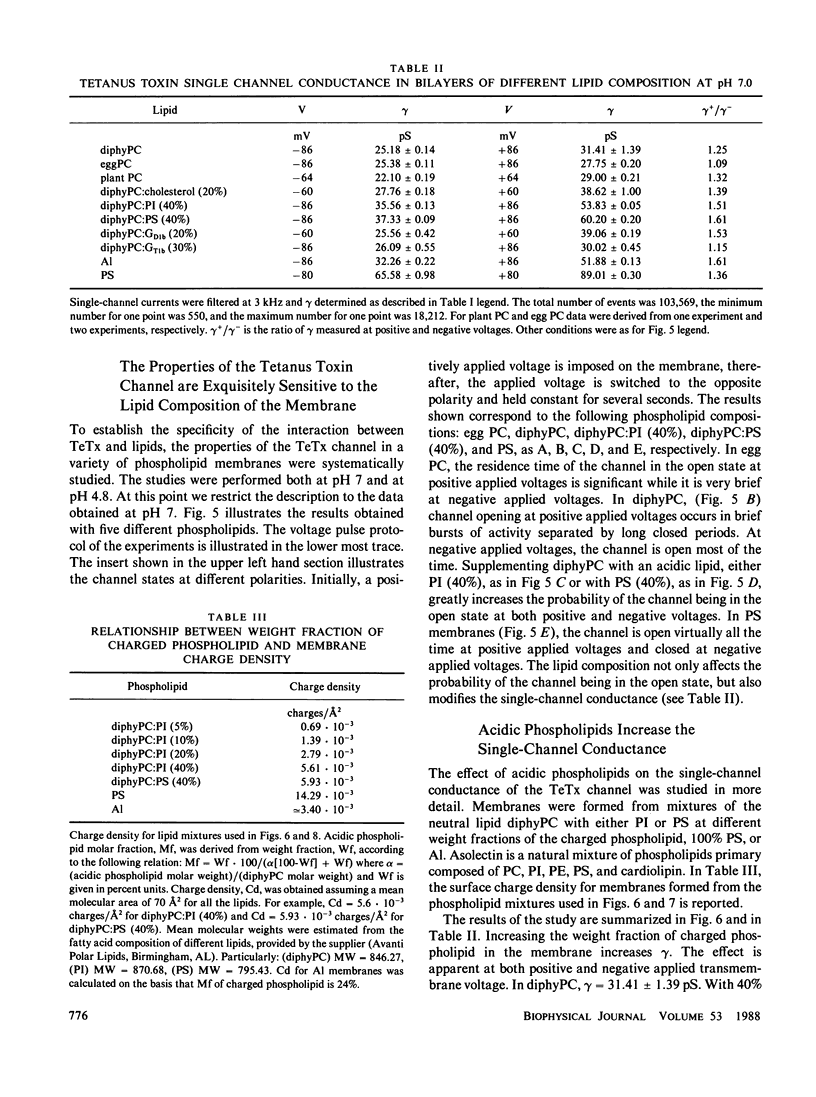
A detailed characterization of the properties of the channel formed by tetanus toxin in planar lipid bilayers is presented. Channel formation proceeds at neutral pH. However, an acidic pH is required to detect the presence of channels in the membrane rapidly and effectively. Acid pH markedly lowers the single-channel conductance, for phosphatidylserine at 0.5 M KCl gamma = 89 pS at pH 7.0 while at pH 4.8, gamma = 30 pS. The toxin channel is cation selective without significant selectivity between potassium and sodium (gamma [K+]/gamma [Na+] greater than or equal to 1.35). In all the lipids studied gamma is larger at positive than at negative voltages. The toxin channel is voltage dependent both at neutral and acidic pH: for phosphatidylserine membranes, the probability of the channel being open is much greater at positive than at negative voltage. In different phospholipids the channel exhibits different voltage dependence. In phosphatidylserine membranes the channel is inactivated at negative voltages, whereas in diphytanoylphosphatidylcholine membranes channels are more active at negative voltages than at positive. The presence of acidic phospholipids in the bilayers increases both the single-channel conductance as well as the probability of the channel being open at positive voltage. A subconductance state is readily identifiable in the single-channel recordings. Accordingly, single-channel conductance histograms are best fitted with a sum of 3 Gaussian distributions corresponding to the closed state, the open subconductance state and the full open state. Channel activity occurs in bursts of openings separated by long closings. Probability density analysis of the open dwell times of the toxin channel indicate the existence of a single open state with a lifetime greater than or equal to 1 ms in all lipids studied. Analysis of intra-bursts closing lifetimes reveals the existence of two components; the slow component is of the order of 1 ms, the fast one is less than or equal to 0.5 ms. The channel activity induced by tetanus toxin in lipid bilayers suggests a mechanism for its neurotoxicity: a voltage dependent, cation selective channel inserted in the postsynaptic membrane would lead to continuous depolarization and, therefore, persistent activation of the postsynaptic cell.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bezanilla F. A high capacity data recording device based on a digital audio processor and a video cassette recorder. Biophys J. 1985 Mar;47(3):437–441. doi: 10.1016/S0006-3495(85)83935-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boquet P., Duflot E., Hauttecoeur B. Low pH induces a hydrophobic domain in the tetanus toxin molecule. Eur J Biochem. 1984 Oct 15;144(2):339–344. doi: 10.1111/j.1432-1033.1984.tb08469.x. [DOI] [PubMed] [Google Scholar]
- Boquet P., Duflot E. Tetanus toxin fragment forms channels in lipid vesicles at low pH. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7614–7618. doi: 10.1073/pnas.79.24.7614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borochov-Neori H., Yavin E., Montal M. Tetanus toxin forms channels in planar lipid bilayers containing gangliosides. Biophys J. 1984 Jan;45(1):83–85. doi: 10.1016/S0006-3495(84)84117-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büttner-Ennever J. A., Grob P., Akert K., Bizzini B. A transsynaptic autoradiographic study of the pathways controlling the extraocular eye muscles, using [125I]B-IIb tetanus toxin fragment. Ann N Y Acad Sci. 1981;374:157–170. doi: 10.1111/j.1749-6632.1981.tb30868.x. [DOI] [PubMed] [Google Scholar]
- Donovan J. J., Middlebrook J. L. Ion-conducting channels produced by botulinum toxin in planar lipid membranes. Biochemistry. 1986 May 20;25(10):2872–2876. doi: 10.1021/bi00358a020. [DOI] [PubMed] [Google Scholar]
- Donovan J. J., Simon M. I., Draper R. K., Montal M. Diphtheria toxin forms transmembrane channels in planar lipid bilayers. Proc Natl Acad Sci U S A. 1981 Jan;78(1):172–176. doi: 10.1073/pnas.78.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan J. J., Simon M. I., Montal M. Insertion of diphtheria toxin into and across membranes: role of phosphoinositide asymmetry. Nature. 1982 Aug 12;298(5875):669–672. doi: 10.1038/298669a0. [DOI] [PubMed] [Google Scholar]
- Donovan J. J., Simon M. I., Montal M. Requirements for the translocation of diphtheria toxin fragment A across lipid membranes. J Biol Chem. 1985 Jul 25;260(15):8817–8823. [PubMed] [Google Scholar]
- Eisel U., Jarausch W., Goretzki K., Henschen A., Engels J., Weller U., Hudel M., Habermann E., Niemann H. Tetanus toxin: primary structure, expression in E. coli, and homology with botulinum toxins. EMBO J. 1986 Oct;5(10):2495–2502. doi: 10.1002/j.1460-2075.1986.tb04527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairweather N. F., Lyness V. A. The complete nucleotide sequence of tetanus toxin. Nucleic Acids Res. 1986 Oct 10;14(19):7809–7812. doi: 10.1093/nar/14.19.7809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman P. H. Role of membrane gangliosides in the binding and action of bacterial toxins. J Membr Biol. 1982;69(2):85–97. doi: 10.1007/BF01872268. [DOI] [PubMed] [Google Scholar]
- Gawade S., Bon C., Bizzini B. The use of antibody Fab fragments specifically directed to two different complementary parts of the tetanus toxin molecule for studying the mode of action of the toxin. Brain Res. 1985 May 13;334(1):139–146. doi: 10.1016/0006-8993(85)90575-x. [DOI] [PubMed] [Google Scholar]
- Helting T. B., Zwisler O. Structure of tetanus toxin. I. Breakdown of the toxin molecule and discrimination between polypeptide fragments. J Biol Chem. 1977 Jan 10;252(1):187–193. [PubMed] [Google Scholar]
- Helting T. B., Zwisler O., Wiegandt H. Structure of tetanus toxin. II. Toxin binding to ganglioside. J Biol Chem. 1977 Jan 10;252(1):194–198. [PubMed] [Google Scholar]
- Hill M. W., Lester R. Mixtures of gangliosides and phosphatidylcholine in aqueous dispersions. Biochim Biophys Acta. 1972 Sep 1;282(1):18–30. doi: 10.1016/0005-2736(72)90307-0. [DOI] [PubMed] [Google Scholar]
- Hoch D. H., Romero-Mira M., Ehrlich B. E., Finkelstein A., DasGupta B. R., Simpson L. L. Channels formed by botulinum, tetanus, and diphtheria toxins in planar lipid bilayers: relevance to translocation of proteins across membranes. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1692–1696. doi: 10.1073/pnas.82.6.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan B. L., Finkelstein A., Colombini M. Diphtheria toxin fragment forms large pores in phospholipid bilayer membranes. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4950–4954. doi: 10.1073/pnas.78.8.4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labarca P., Lindstrom J., Montal M. Acetylcholine receptor in planar lipid bilayers. Characterization of the channel properties of the purified nicotinic acetylcholine receptor from Torpedo californica reconstituted in planar lipid bilayers. J Gen Physiol. 1984 Apr;83(4):473–496. doi: 10.1085/jgp.83.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledley F. D., Lee G., Kohn L. D., Habig W. H., Hardegree M. C. Tetanus toxin interactions with thyroid plasma membranes. Implications for structure and function of tetanus toxin receptors and potential pathophysiological significance. J Biol Chem. 1977 Jun 25;252(12):4049–4055. [PubMed] [Google Scholar]
- Maggio B., Cumar F. A., Caputto R. Molecular behaviour of glycosphingolipids in interfaces. Possible participation in some properties of nerve membranes. Biochim Biophys Acta. 1981 Dec;650(2-3):69–87. doi: 10.1016/0304-4157(81)90001-0. [DOI] [PubMed] [Google Scholar]
- Matsuda M., Yoneda M. Isolation and purification of two antigenically active, "complimentary" polypeptide fragments of tetanus neurotoxin. Infect Immun. 1975 Nov;12(5):1147–1153. doi: 10.1128/iai.12.5.1147-1153.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel R. V., Sharp K., Brooks D., McLaughlin A. C., Winiski A. P., Cafiso D., McLaughlin S. Electrokinetic and electrostatic properties of bilayers containing gangliosides GM1, GD1a, or GT1. Comparison with a nonlinear theory. Biophys J. 1986 Mar;49(3):741–752. doi: 10.1016/S0006-3495(86)83700-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel R., McLaughlin S. The interaction of calcium with gangliosides in bilayer membranes. Biochim Biophys Acta. 1985 Oct 10;819(2):153–160. doi: 10.1016/0005-2736(85)90169-5. [DOI] [PubMed] [Google Scholar]
- McLaughlin S. G., Szabo G., Eisenman G. Divalent ions and the surface potential of charged phospholipid membranes. J Gen Physiol. 1971 Dec;58(6):667–687. doi: 10.1085/jgp.58.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellanby J., Green J. How does tetanus toxin act? Neuroscience. 1981;6(3):281–300. doi: 10.1016/0306-4522(81)90123-8. [DOI] [PubMed] [Google Scholar]
- Montal M. Formation of bimolecular membranes from lipid monolayers. Methods Enzymol. 1974;32:545–554. doi: 10.1016/0076-6879(74)32053-8. [DOI] [PubMed] [Google Scholar]
- Montecucco C., Schiavo G., Brunner J., Duflot E., Boquet P., Roa M. Tetanus toxin is labeled with photoactivatable phospholipids at low pH. Biochemistry. 1986 Feb 25;25(4):919–924. doi: 10.1021/bi00352a027. [DOI] [PubMed] [Google Scholar]
- Montesano R., Roth J., Robert A., Orci L. Non-coated membrane invaginations are involved in binding and internalization of cholera and tetanus toxins. Nature. 1982 Apr 15;296(5858):651–653. doi: 10.1038/296651a0. [DOI] [PubMed] [Google Scholar]
- Neubauer V., Helting T. B. Structure of tetanus toxin: the arrangement of papain digestion products within the heavy chain-light chain framework of extracellular toxin. Biochim Biophys Acta. 1981 Mar 27;668(1):141–148. doi: 10.1016/0005-2795(81)90157-4. [DOI] [PubMed] [Google Scholar]
- O'Brien D. F., Costa L. F., Ott R. A. Photochemical functionality of rhodopsin-phospholipid recombinant membranes. Biochemistry. 1977 Apr 5;16(7):1295–1303. doi: 10.1021/bi00626a009. [DOI] [PubMed] [Google Scholar]
- Penner R., Neher E., Dreyer F. Intracellularly injected tetanus toxin inhibits exocytosis in bovine adrenal chromaffin cells. Nature. 1986 Nov 6;324(6092):76–78. doi: 10.1038/324076a0. [DOI] [PubMed] [Google Scholar]
- Prod'hom B., Pietrobon D., Hess P. Direct measurement of proton transfer rates to a group controlling the dihydropyridine-sensitive Ca2+ channel. Nature. 1987 Sep 17;329(6136):243–246. doi: 10.1038/329243a0. [DOI] [PubMed] [Google Scholar]
- Roa M., Boquet P. Interaction of tetanus toxin with lipid vesicles at low pH. Protection of specific polypeptides against proteolysis. J Biol Chem. 1985 Jun 10;260(11):6827–6835. [PubMed] [Google Scholar]
- Schwab M. E., Suda K., Thoenen H. Selective retrograde transsynaptic transfer of a protein, tetanus toxin, subsequent to its retrograde axonal transport. J Cell Biol. 1979 Sep;82(3):798–810. doi: 10.1083/jcb.82.3.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson L. L. Molecular pharmacology of botulinum toxin and tetanus toxin. Annu Rev Pharmacol Toxicol. 1986;26:427–453. doi: 10.1146/annurev.pa.26.040186.002235. [DOI] [PubMed] [Google Scholar]
- Usai C., Marchetti C., Gambale F., Robello M., Gorio A. Capacitance--voltage relationship in phospholipid bilayers containing gangliosides. FEBS Lett. 1983 Mar 21;153(2):315–319. doi: 10.1016/0014-5793(83)80632-2. [DOI] [PubMed] [Google Scholar]
- VAN HEYNINGEN W. E., MILLER P. A. The fixation of tetanus toxin by ganglioside. J Gen Microbiol. 1961 Jan;24:107–119. doi: 10.1099/00221287-24-1-107. [DOI] [PubMed] [Google Scholar]
- Yavin Z., Yavin E., Kohn L. D. Sequestration of tetanus toxin in developing neuronal cell cultures. J Neurosci Res. 1982;7(3):267–278. doi: 10.1002/jnr.490070304. [DOI] [PubMed] [Google Scholar]


