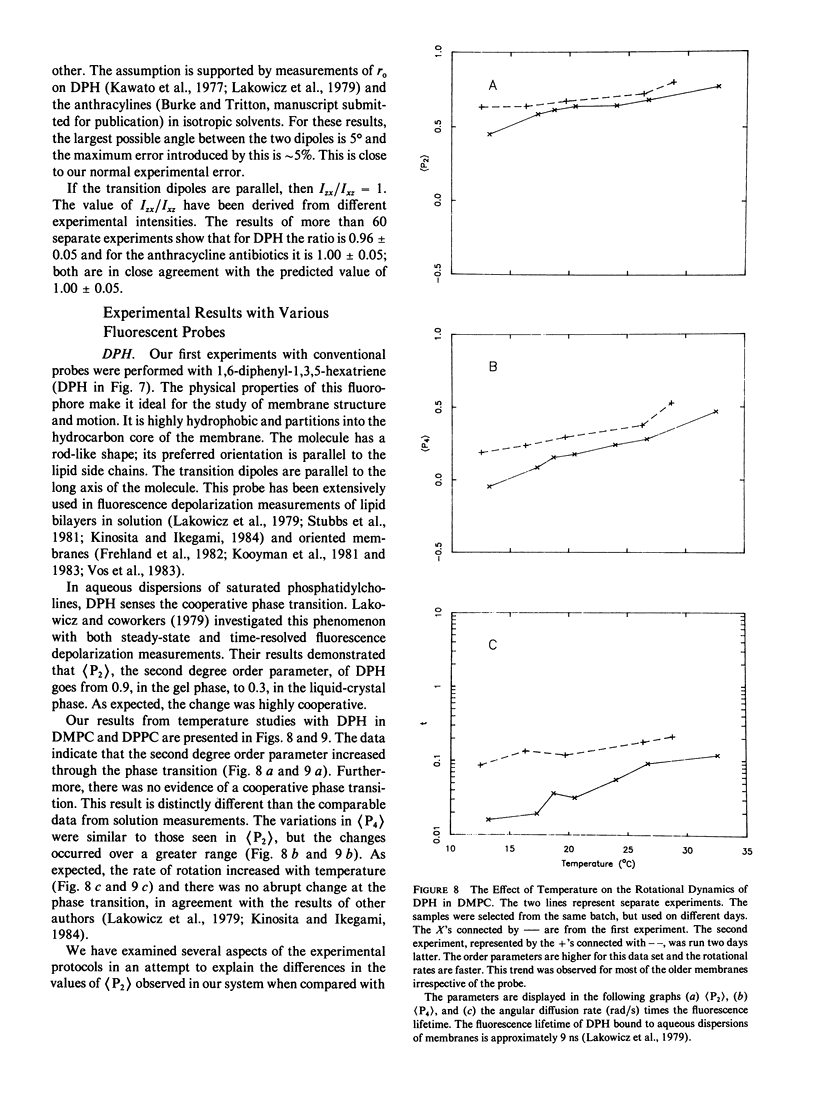
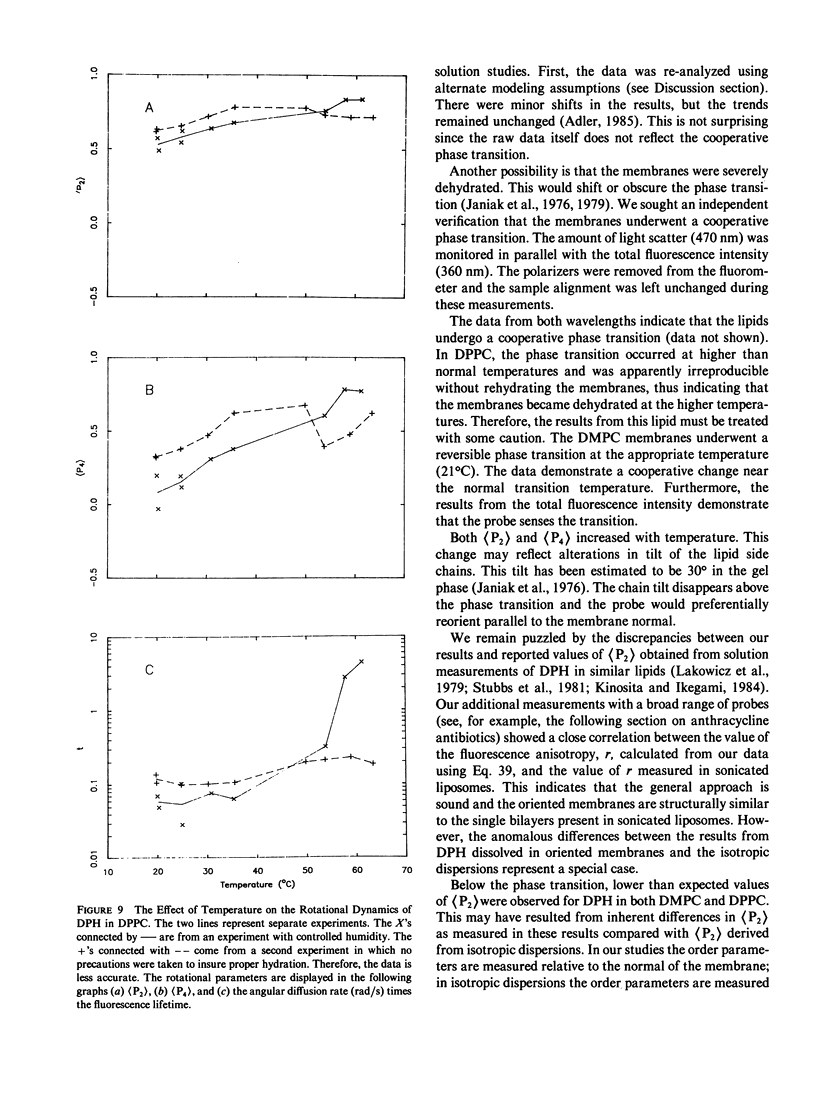
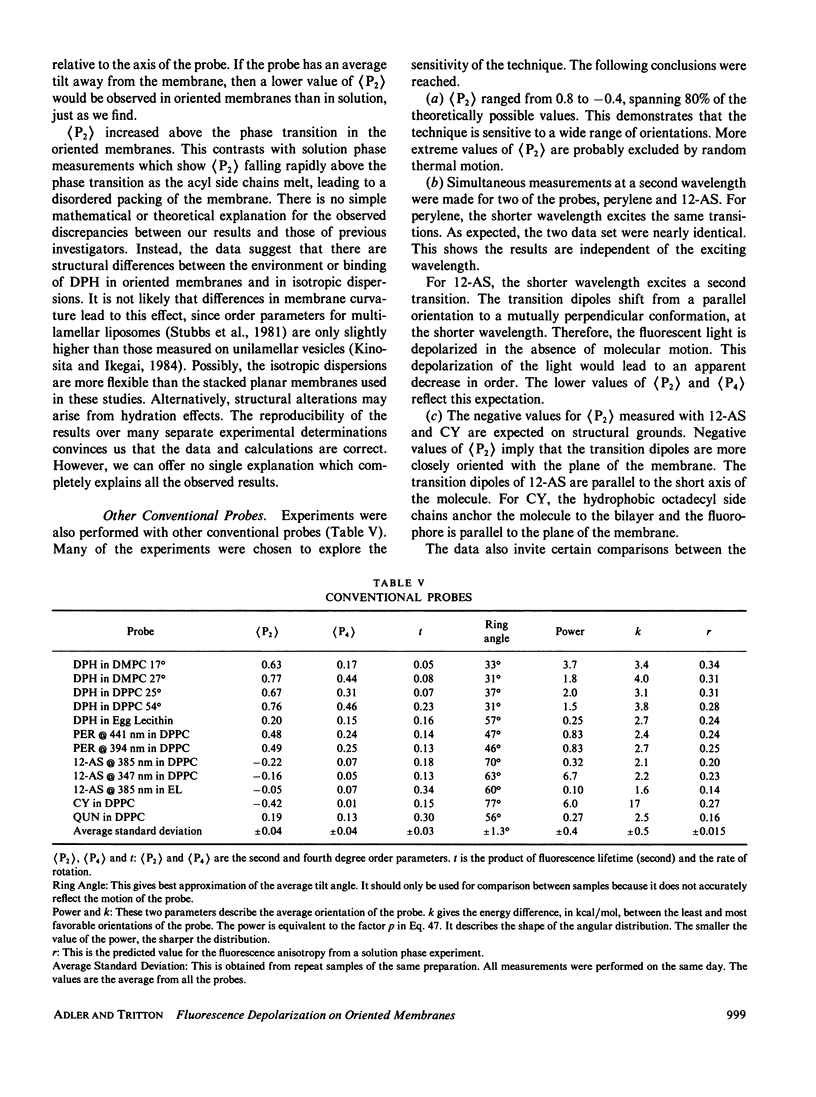
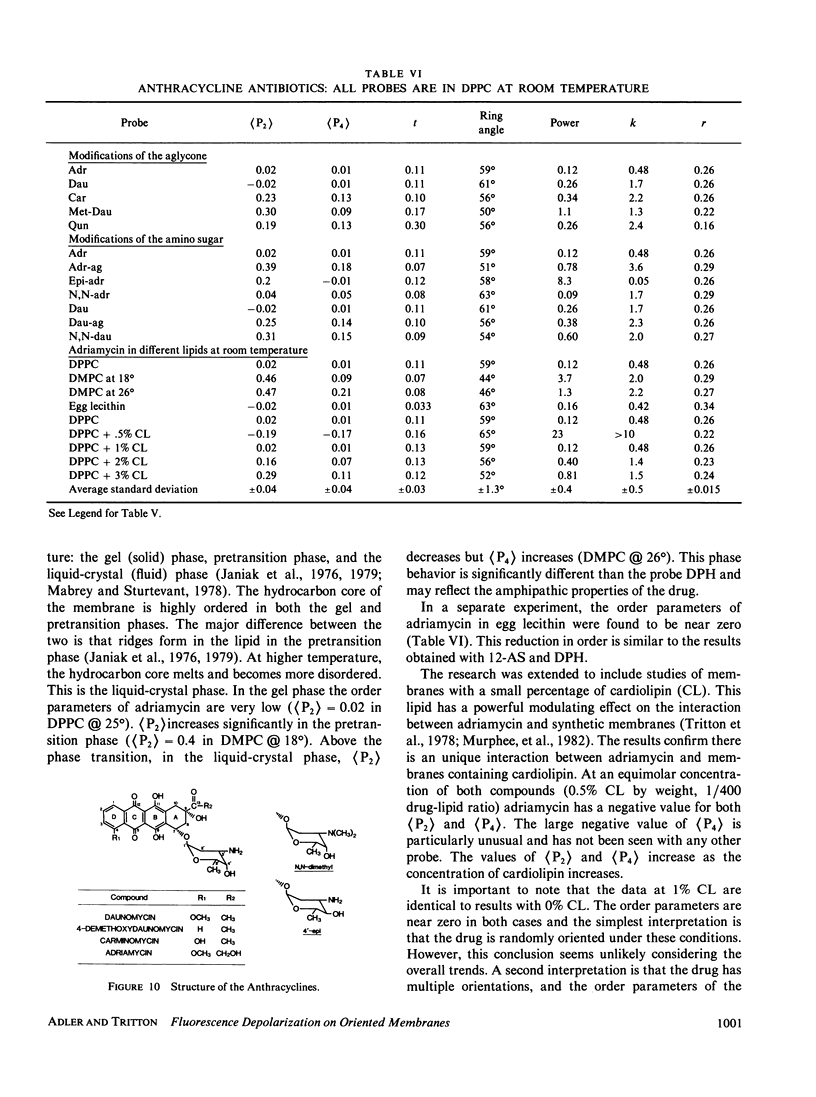
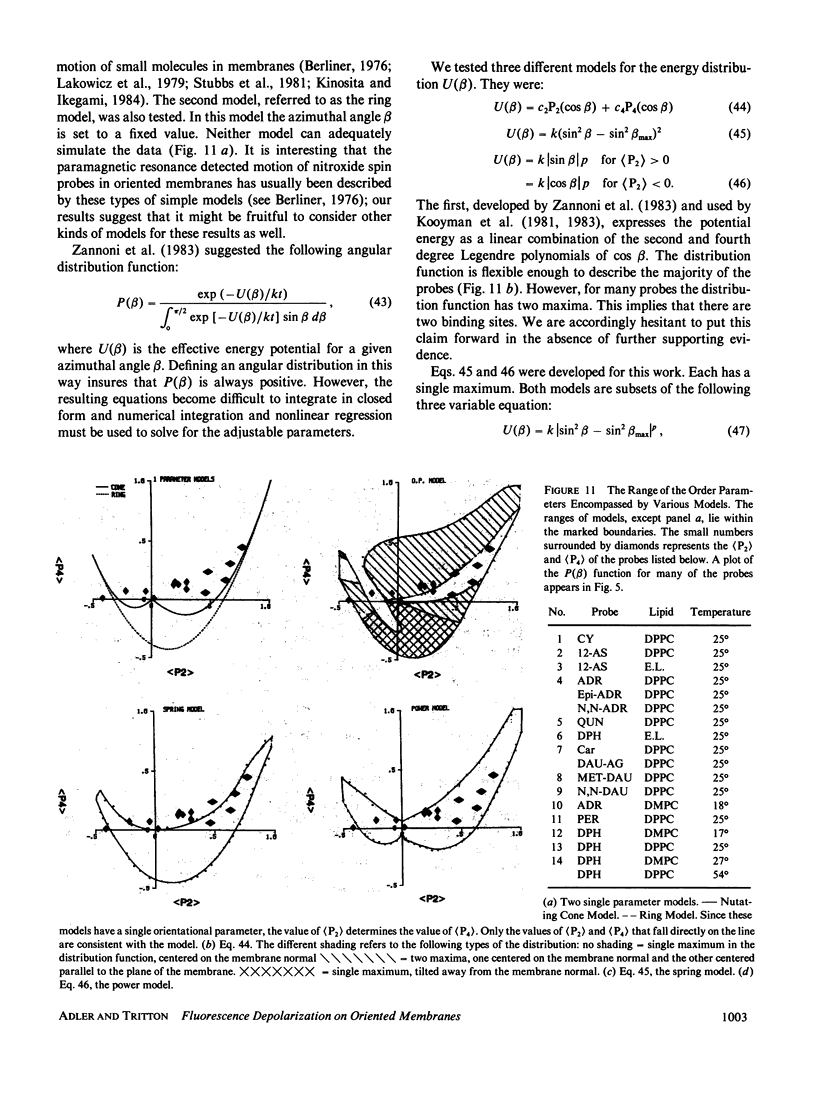
Abstract
We describe the theory and experimental application of fluorescence depolarization measurements on small molecules bound to oriented phospholipid bilayers. The results yield insight into both the orientation and the rotational motion of fluorophores in a membrane environment. To accomplish this the angular distribution of polarized fluorescence intensities is measured on a membrane preparation consisting of stacked phospholipid bilayers oriented in a known coordinate system. Considerably more information is available from this data than in comparable solution phase measurements. Three parameters are derived from the data: the rate of rotational diffusion and the second and fourth degree order parameters. These latter two parameters provide an assessment of the average distribution of fluorophore orientation in the membrane bilayer. The data have been carefully examined for systematic experimental artifacts and new protocols are presented which help to eliminate errors that have not been amply treated in the past. We present data for two types of fluorescent molecules: (a) conventional membrane probes like diphenylhexatriene, perylene and anthroyloxy fatty acids; and (b) the anticancer agent adriamycin and several congeneric anthracycline antibiotics. The results show that the hydrocarbon core of membranes is more rigid than previously thought, particularly above the thermal phase transition temperature. We also show that the orientation of small molecules is sensitive to both the phospholipid composition and to the interaction of specific functional groups with the lipid bilayer. The results are discussed in terms of energetic models describing the general patterns for the binding of small molecules to biological membranes.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badley R. A., Martin W. G., Schneider H. Dynamic behavior of fluorescent probes in lipid bilayer model membranes. Biochemistry. 1973 Jan 16;12(2):268–275. doi: 10.1021/bi00726a015. [DOI] [PubMed] [Google Scholar]
- Badley R. A., Schneider H., Martin W. G. Orientation and motion of a fluorescent probe in model membranes. Biochem Biophys Res Commun. 1971 Oct 1;45(1):174–183. doi: 10.1016/0006-291x(71)90066-0. [DOI] [PubMed] [Google Scholar]
- Burke T. G., Tritton T. R. Location and dynamics of anthracyclines bound to unilamellar phosphatidylcholine vesicles. Biochemistry. 1985 Oct 8;24(21):5972–5980. doi: 10.1021/bi00342a043. [DOI] [PubMed] [Google Scholar]
- Burke T. G., Tritton T. R. Structural basis of anthracycline selectivity for unilamellar phosphatidylcholine vesicles: an equilibrium binding study. Biochemistry. 1985 Mar 26;24(7):1768–1776. doi: 10.1021/bi00328a030. [DOI] [PubMed] [Google Scholar]
- Cherry R. J., Chapman D. Optical properties of black lecithin films. J Mol Biol. 1969 Feb 28;40(1):19–32. doi: 10.1016/0022-2836(69)90293-9. [DOI] [PubMed] [Google Scholar]
- Frehland E., Kreikenbohm R., Pohl W. G. Steady-state fluorescence polarization in planar lipid membranes. Experimental and theoretical analysis of the fluorophores 8-anilino-1-naphthalenesulfonate, 1,6-Diphenyl-1,3,5-hexatriene, dansyllysine-valinomycin and n-(9-anthroyloxy) fatty acids. Biophys Chem. 1982 Apr;15(1):73–86. doi: 10.1016/0301-4622(82)87018-x. [DOI] [PubMed] [Google Scholar]
- Janiak M. J., Small D. M., Shipley G. G. Nature of the Thermal pretransition of synthetic phospholipids: dimyristolyl- and dipalmitoyllecithin. Biochemistry. 1976 Oct 19;15(21):4575–4580. doi: 10.1021/bi00666a005. [DOI] [PubMed] [Google Scholar]
- Janiak M. J., Small D. M., Shipley G. G. Temperature and compositional dependence of the structure of hydrated dimyristoyl lecithin. J Biol Chem. 1979 Jul 10;254(13):6068–6078. [PubMed] [Google Scholar]
- Kawato S., Kinosita K., Jr, Ikegami A. Dynamic structure of lipid bilayers studied by nanosecond fluorescence techniques. Biochemistry. 1977 May 31;16(11):2319–2324. doi: 10.1021/bi00630a002. [DOI] [PubMed] [Google Scholar]
- Lakowicz J. R., Knutson J. R. Hindered depolarizing rotations of perylene in lipid bilayers. Detection by lifetime-resolved fluorescence anisotropy measurements. Biochemistry. 1980 Mar 4;19(5):905–911. doi: 10.1021/bi00546a013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakowicz J. R., Prendergast F. G., Hogen D. Differential polarized phase fluorometric investigations of diphenylhexatriene in lipid bilayers. Quantitation of hindered depolarizing rotations. Biochemistry. 1979 Feb 6;18(3):508–519. doi: 10.1021/bi00570a021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphree S. A., Murphy D., Sartorelli A. C., Tritton T. R. Adriamycin-liposome interactions. A magnetic resonance study of the differential effects of cardiolipin on drug-induced fusion and permeability. Biochim Biophys Acta. 1982 Sep 24;691(1):97–105. doi: 10.1016/0005-2736(82)90218-8. [DOI] [PubMed] [Google Scholar]
- Rogers K. E., Carr B. I., Tökés Z. A. Cell surface-mediated cytotoxicity of polymer-bound Adriamycin against drug-resistant hepatocytes. Cancer Res. 1983 Jun;43(6):2741–2748. [PubMed] [Google Scholar]
- Stubbs C. D., Kouyama T., Kinosita K., Jr, Ikegami A. Effect of double bonds on the dynamic properties of the hydrocarbon region of lecithin bilayers. Biochemistry. 1981 Jul 21;20(15):4257–4262. doi: 10.1021/bi00518a004. [DOI] [PubMed] [Google Scholar]
- Tokes Z. A., Rogers K. E., Rembaum A. Synthesis of adriamycin-coupled polyglutaraldehyde microspheres and evaluation of their cytostatic activity. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2026–2030. doi: 10.1073/pnas.79.6.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triton T. R., Yee G. The anticancer agent adriamycin can be actively cytotoxic without entering cells. Science. 1982 Jul 16;217(4556):248–250. doi: 10.1126/science.7089561. [DOI] [PubMed] [Google Scholar]
- Tritton T. R., Murphree S. A., Sartorelli A. C. Adriamycin: a proposal on the specificity of drug action. Biochem Biophys Res Commun. 1978 Oct 16;84(3):802–808. doi: 10.1016/0006-291x(78)90775-1. [DOI] [PubMed] [Google Scholar]
- Tritton T. R., Yee G., Wingard L. B., Jr Immobilized adriamycin: a tool for separating cell surface from intracellular mechanisms. Fed Proc. 1983 Feb;42(2):284–287. [PubMed] [Google Scholar]
- Vos M. H., Kooyman R. P., Levine Y. K. Angle resolved fluorescence depolarization experiments on oriented lipid membrane systems. Biochem Biophys Res Commun. 1983 Oct 31;116(2):462–468. doi: 10.1016/0006-291x(83)90546-6. [DOI] [PubMed] [Google Scholar]
- Wingard L. B., Jr, Tritton T. R., Egler K. A. Cell surface effects of adriamycin and carminomycin immobilized on cross-linked polyvinyl alcohol. Cancer Res. 1985 Aug;45(8):3529–3536. [PubMed] [Google Scholar]
- Yguerabide J., Stryer L. Fluorescence spectroscopy of an oriented model membrane. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1217–1221. doi: 10.1073/pnas.68.6.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. C., Ozols R. F., Myers C. E. The anthracycline antineoplastic drugs. N Engl J Med. 1981 Jul 16;305(3):139–153. doi: 10.1056/NEJM198107163050305. [DOI] [PubMed] [Google Scholar]



