Abstract
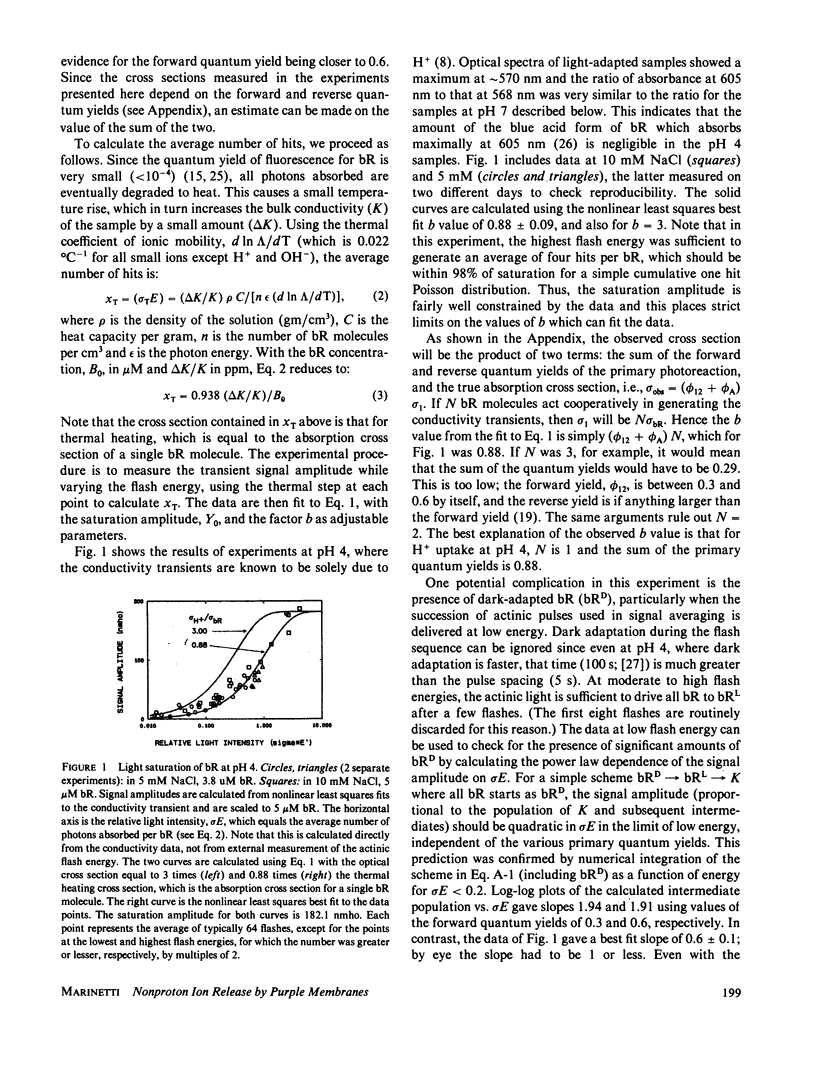
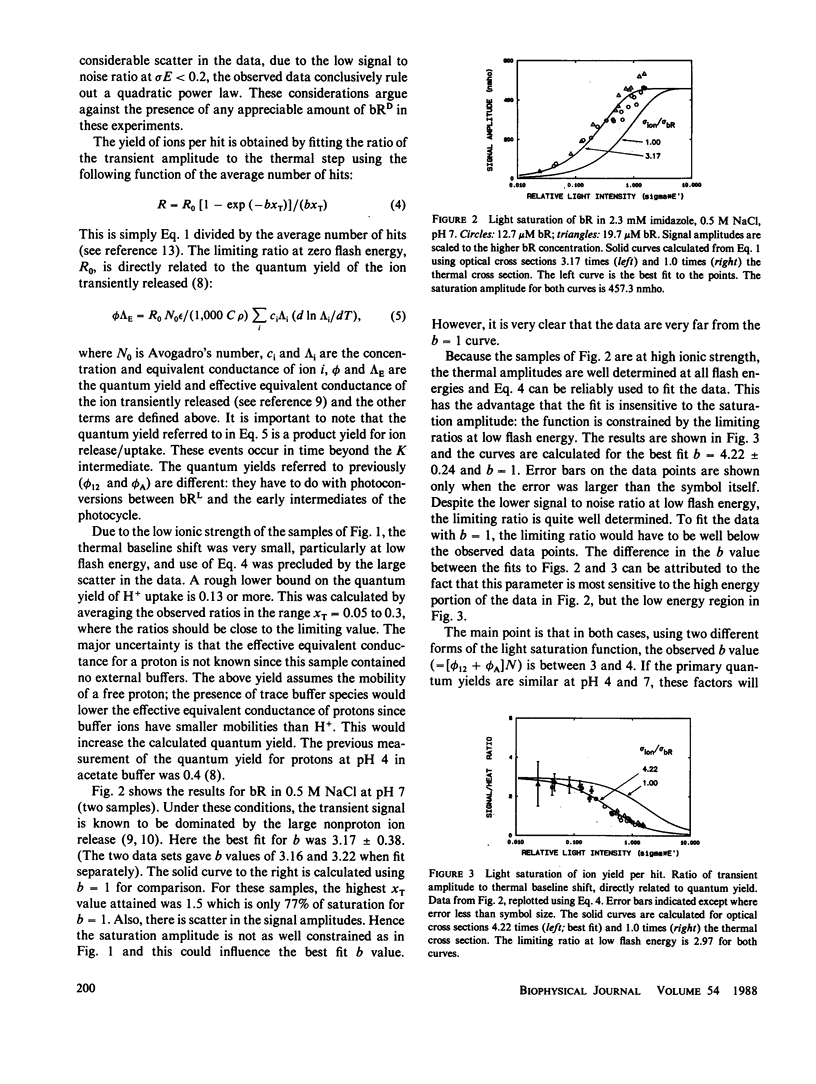
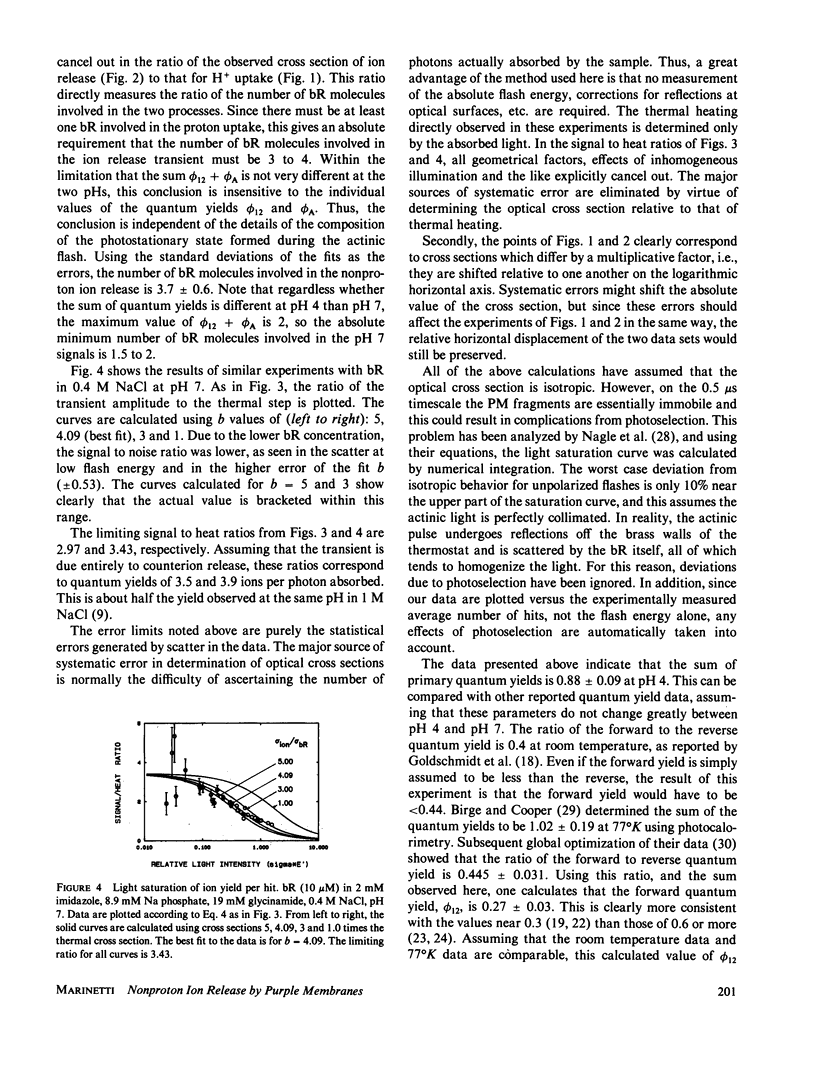
The amplitudes of the conductivity transients in photoexcited purple membranes were studied as a function of the energy of the actinic flash to determine the optical cross section of the process giving rise to the conductivity transient. Heating of the solution by the absorbed light causes an additional conductivity change and serves as an internal actinometer; the experiment directly yields the ratio of the cross section of ion release/uptake to that for light absorption. In effect, this counts the number of bacteriorhodopsin (bR) molecules involved in the conductivity transient per photon absorbed. At pH 7 in 0.4-0.5 M NaCl, where the conductivity signals are dominated by nonproton ions, the ratio is between 3 and 4, i.e., excitation of any one of several chromophores generates the same ion release signal. The simplest interpretation is that at pH 7 cooperative conformational changes cause a transient change in the surface charge distribution near all the affected bR molecules, resulting in the transient release of numerous counterions. As a comparison, at pH 4 where the signals are due to protons alone, the cross section data indicate that only a single bR molecule is involved in the proton movements. In this case, the results also show that the sum of the primary forward and reverse quantum yields (for the reactions: bR----K) is 0.88 +/- 0.09.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahl P. L., Cone R. A. Light activates rotations of bacteriorhodopsin in the purple membrane. Biophys J. 1984 Jun;45(6):1039–1049. doi: 10.1016/S0006-3495(84)84251-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becher B., Ebrey T. G. Evidence for chromophore-chromophore (exciton) interaction in the purple membrane of Halobacterium halobium. Biochem Biophys Res Commun. 1976 Mar 8;69(1):1–6. doi: 10.1016/s0006-291x(76)80263-x. [DOI] [PubMed] [Google Scholar]
- Birge R. R., Cooper T. M. Energy storage in the primary step of the photocycle of bacteriorhodopsin. Biophys J. 1983 Apr;42(1):61–69. doi: 10.1016/S0006-3495(83)84369-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dencher N. A., Heyn M. P. Bacteriorhodopsin monomers pump protons. FEBS Lett. 1979 Dec 15;108(2):307–310. doi: 10.1016/0014-5793(79)80552-9. [DOI] [PubMed] [Google Scholar]
- Goldschmidt C. R., Kalisky O., Rosenfeld T., Ottolenghi M. The quantum efficiency of the bacteriorhodopsin photocycle. Biophys J. 1977 Feb;17(2):179–183. doi: 10.1016/S0006-3495(77)85635-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt C. R., Ottolenghi M., Korenstein R. On the primary quantum yields in the bacteriorhodopsin photocycle. Biophys J. 1976 Jul;16(7):839–843. doi: 10.1016/S0006-3495(76)85732-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindjee R., Becher B., Ebrey T. G. The fluorescence from the chromophore of the purple membrane protein. Biophys J. 1978 Apr;22(1):67–77. doi: 10.1016/S0006-3495(78)85471-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson R. The purple membrane from Halobacterium halobium. Annu Rev Biophys Bioeng. 1977;6:87–109. doi: 10.1146/annurev.bb.06.060177.000511. [DOI] [PubMed] [Google Scholar]
- Heyn M. P., Bauer P. J., Dencher N. A. A natural CD label to probe the structure of the purple membrane from Halobacterium halobium by means of exciton coupling effects. Biochem Biophys Res Commun. 1975 Dec 1;67(3):897–903. doi: 10.1016/0006-291x(75)90761-5. [DOI] [PubMed] [Google Scholar]
- Hurley J. B., Ebrey T. G. Energy transfer in the purple membrane of Halobacterium halobium. Biophys J. 1978 Apr;22(1):49–66. doi: 10.1016/S0006-3495(78)85470-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozier R. H., Bogomolni R. A., Stoeckenius W. Bacteriorhodopsin: a light-driven proton pump in Halobacterium Halobium. Biophys J. 1975 Sep;15(9):955–962. doi: 10.1016/S0006-3495(75)85875-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinetti T. Abrupt onset of large scale nonproton ion release in purple membranes caused by increasing pH or ionic strength. Biophys J. 1987 Jun;51(6):875–881. doi: 10.1016/S0006-3495(87)83415-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinetti T. Large scale nonproton ion release and bacteriorhodopsin's state of aggregation in lipid vesicles. I. Monomers. Biophys J. 1987 Jul;52(1):115–121. doi: 10.1016/S0006-3495(87)83195-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinetti T., Mauzerall D. Absolute quantum yields and proof of proton and nonproton transient release and uptake in photoexcited bacteriorhodopsin. Proc Natl Acad Sci U S A. 1983 Jan;80(1):178–180. doi: 10.1073/pnas.80.1.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinetti T., Mauzerall D. Large transient nonproton ion movements in purple membrane suspensions are abolished by solubilization in Triton X-100. Biophys J. 1986 Sep;50(3):405–415. doi: 10.1016/S0006-3495(86)83476-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowery P. C., Lozier R. H., Chae Q., Tseng Y. W., Taylor M., Stoeckenius W. Effect of acid pH on the absorption spectra and photoreactions of bacteriorhodopsin. Biochemistry. 1979 Sep 18;18(19):4100–4107. doi: 10.1021/bi00586a007. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Hegemann P., Tittor J. The photocycle of the chloride pump halorhodopsin. II: Quantum yields and a kinetic model. EMBO J. 1985 Sep;4(9):2351–2356. doi: 10.1002/j.1460-2075.1985.tb03938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterhelt D., Hess B. Reversible photolysis of the purple complex in the purple membrane of Halobacterium halobium. Eur J Biochem. 1973 Aug 17;37(2):316–326. doi: 10.1111/j.1432-1033.1973.tb02990.x. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Functions of a new photoreceptor membrane. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2853–2857. doi: 10.1073/pnas.70.10.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno K., Takeuchi Y., Yoshida M. Effect of light-adaptation on the photoreaction of bacteriorhodopsin from Halobacterium halobium. Biochim Biophys Acta. 1977 Dec 23;462(3):575–582. doi: 10.1016/0005-2728(77)90102-5. [DOI] [PubMed] [Google Scholar]
- Polland H. J., Franz M. A., Zinth W., Kaiser W., Kölling E., Oesterhelt D. Early picosecond events in the photocycle of bacteriorhodopsin. Biophys J. 1986 Mar;49(3):651–662. doi: 10.1016/S0006-3495(86)83692-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehorek M., Heyn M. P. Binding of all-trans-retinal to the purple membrane. Evidence for cooperativity and determination of the extinction coefficient. Biochemistry. 1979 Oct 30;18(22):4977–4983. doi: 10.1021/bi00589a027. [DOI] [PubMed] [Google Scholar]
- Slifkin M. A., Garty H., Sherman W. V., Vincent M. F., Caplan S. R. Light-induced conductivity changes in purple membrane suspensions. Biophys Struct Mech. 1979 Aug;5(4):313–320. doi: 10.1007/BF02426665. [DOI] [PubMed] [Google Scholar]
- Stoeckenius W., Lozier R. H. Light energy conversion in Halobacterium halobium. J Supramol Struct. 1974;2(5-6):769–774. doi: 10.1002/jss.400020519. [DOI] [PubMed] [Google Scholar]
- Xie A. H., Nagle J. F., Lozier R. H. Flash spectroscopy of purple membrane. Biophys J. 1987 Apr;51(4):627–635. doi: 10.1016/S0006-3495(87)83387-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


