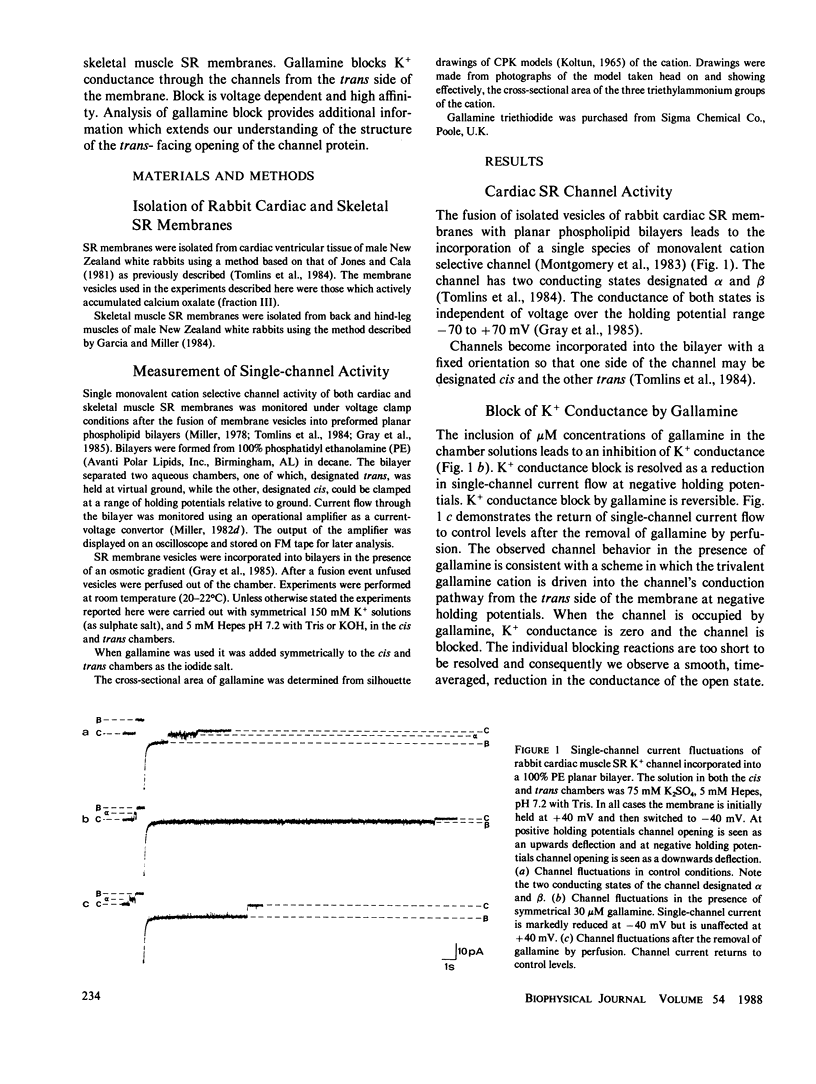
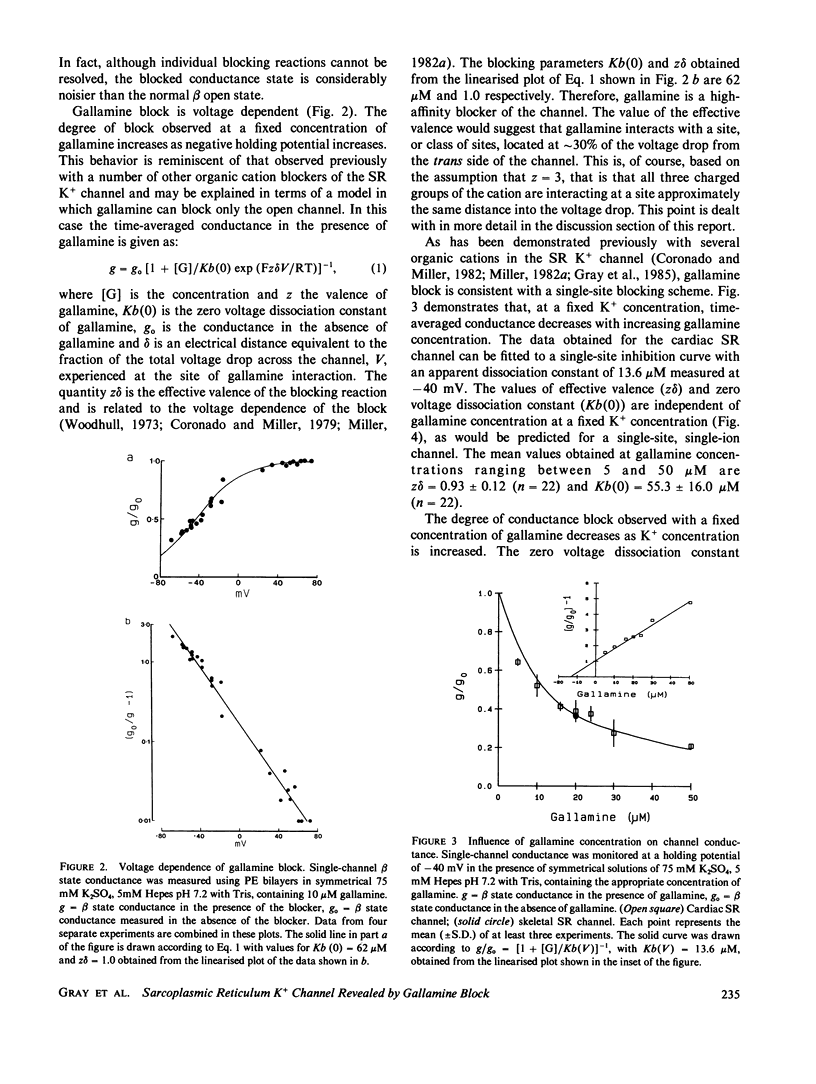
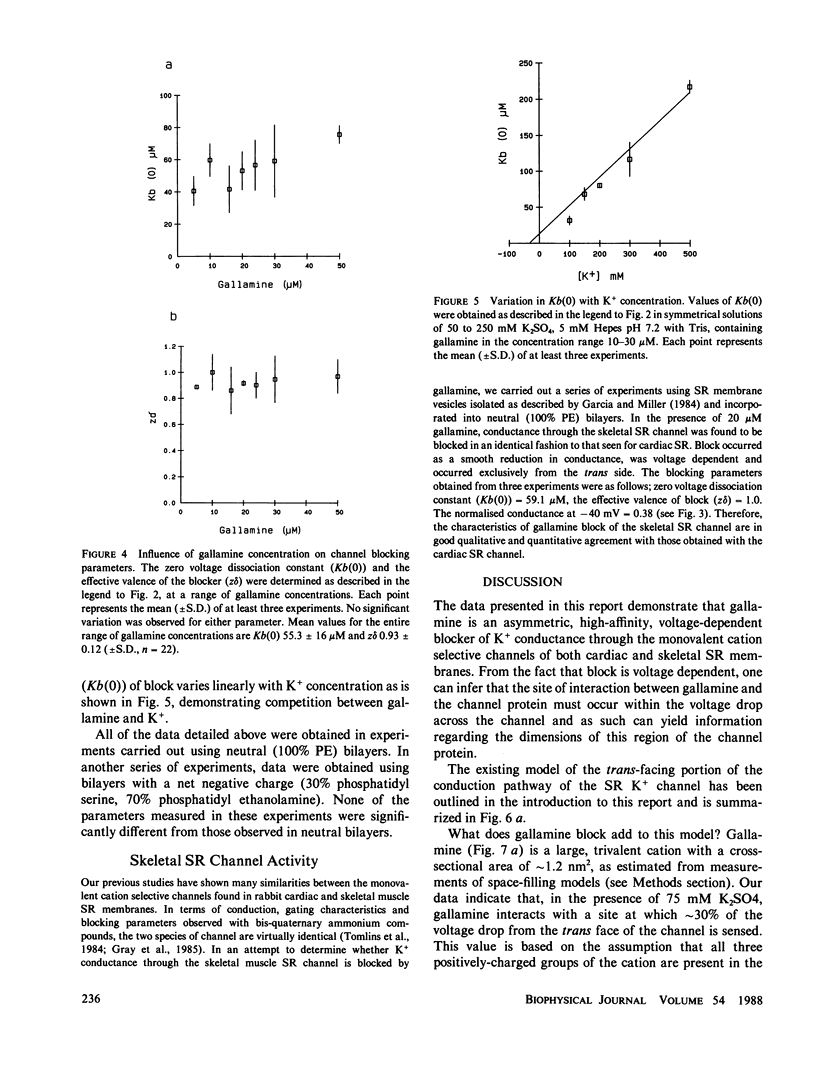
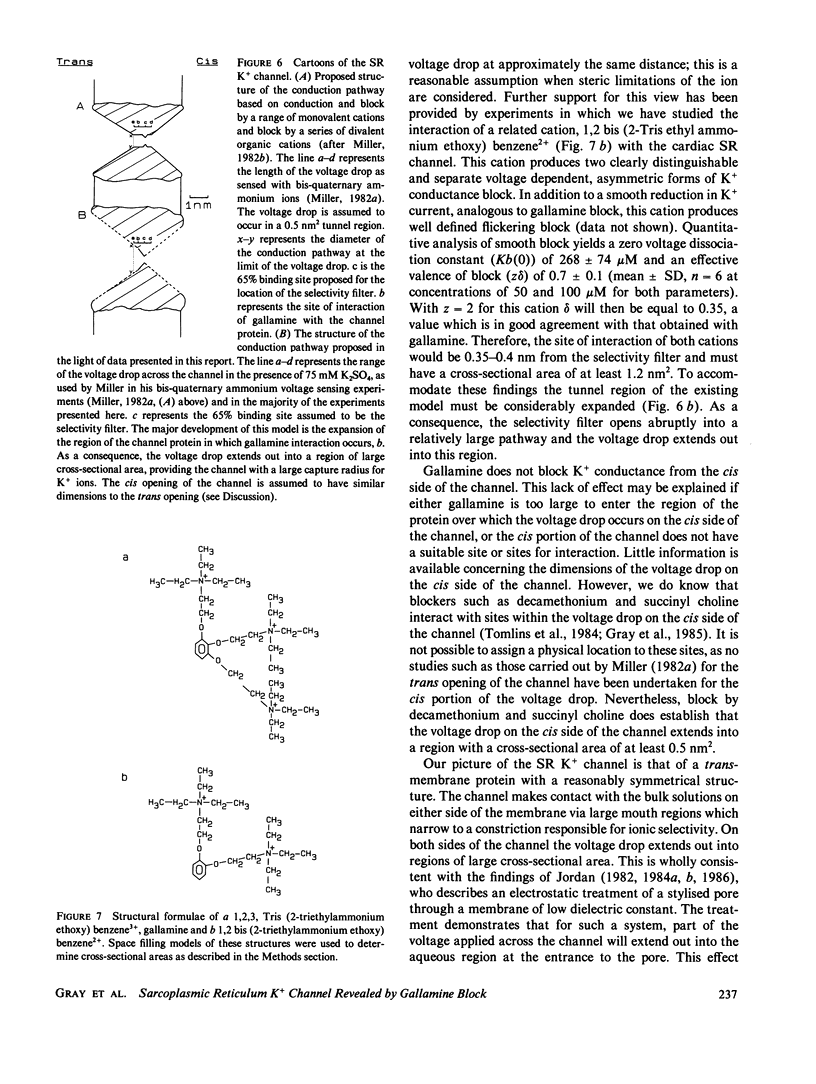
Abstract
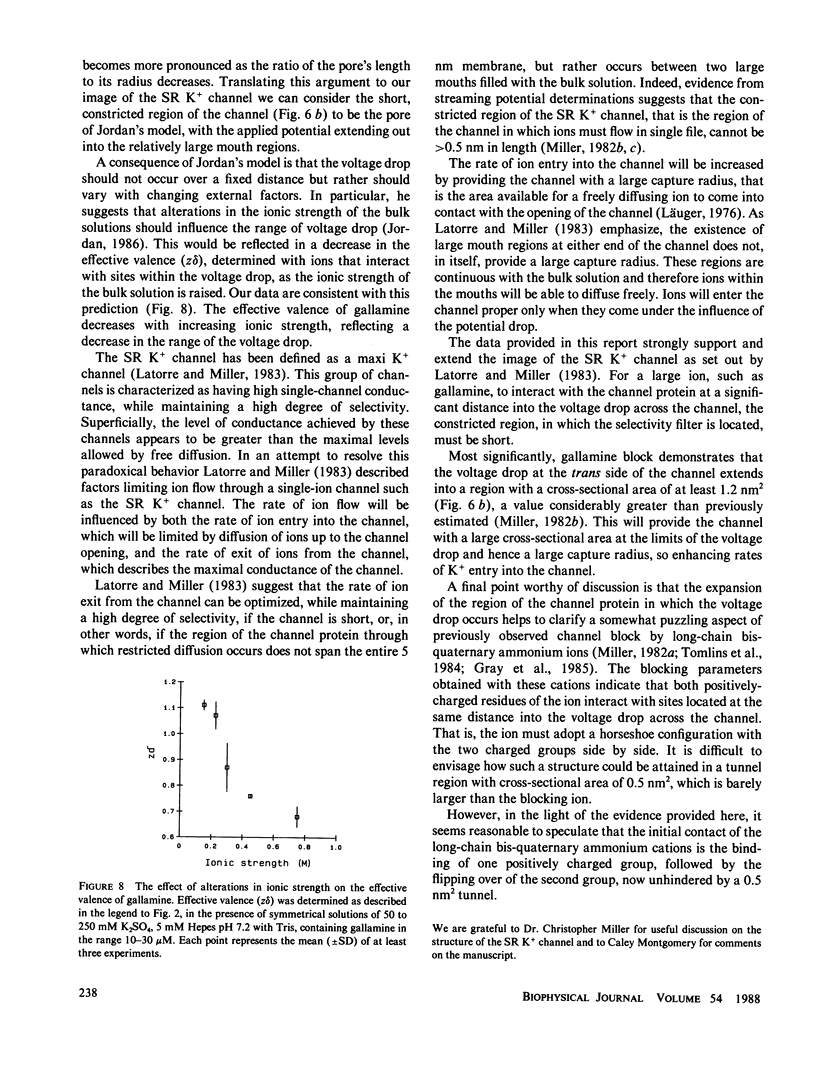
We have studied single-channel conductance fluctuations of K+ channels present in the sarcoplasmic reticulum (SR) membrane systems of rabbit cardiac and skeletal muscle. K+ conductance through the channels is reversibly blocked by gallamine. Conductance block occurs only from the trans side of the channel and is resolved as a smooth reduction in the open state conductance. At a fixed K+ concentration, conduction decreases with increasing gallamine concentration and the data can be fitted to a single-site inhibition scheme. The degree of block seen at a constant gallamine concentration decreases as K+ concentration is increased, indicating competition between gallamine and K+. Gallamine block is voltage dependent, the degree of block increasing with increasing negative holding potential. Quantitative analysis of block yields a zero voltage dissociation constant of 55.3 +/- 16 microM and an effective valence of block of 0.93 +/- 0.12. We conclude that gallamine blocks by interacting with a site or sites located at an electrical distance 30-35% into the voltage drop from the trans side of the channel. This site must have a cross-sectional area of at least 1.2 nm2. The results of this study have been used to modify and extend our view of the structure of the channel's conduction pathway.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Coronado R., Miller C. Conduction and block by organic cations in a K+-selective channel from sarcoplasmic reticulum incorporated into planar phospholipid bilayers. J Gen Physiol. 1982 Apr;79(4):529–547. doi: 10.1085/jgp.79.4.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French R. J., Shoukimas J. J. Blockage of squid axon potassium conductance by internal tetra-N-alkylammonium ions of various sizes. Biophys J. 1981 May;34(2):271–291. doi: 10.1016/S0006-3495(81)84849-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia A. M., Miller C. Channel-mediated monovalent cation fluxes in isolated sarcoplasmic reticulum vesicles. J Gen Physiol. 1984 Jun;83(6):819–839. doi: 10.1085/jgp.83.6.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray M. A., Montgomery R. A., Williams A. J. Asymmetric block of a monovalent cation-selective channel of rabbit cardiac sarcoplasmic reticulum by succinyl choline. J Membr Biol. 1985;88(1):85–95. doi: 10.1007/BF01871216. [DOI] [PubMed] [Google Scholar]
- Hille B. Ionic selectivity of Na and K channels of nerve membranes. Membranes. 1975;3:255–323. [PubMed] [Google Scholar]
- Hille B. Potassium channels in myelinated nerve. Selective permeability to small cations. J Gen Physiol. 1973 Jun;61(6):669–686. doi: 10.1085/jgp.61.6.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. The permeability of the sodium channel to organic cations in myelinated nerve. J Gen Physiol. 1971 Dec;58(6):599–619. doi: 10.1085/jgp.58.6.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L. Y., Catterall W. A., Ehrenstein G. Selectivity of cations and nonelectrolytes for acetylcholine-activated channels in cultured muscle cells. J Gen Physiol. 1978 Apr;71(4):397–410. doi: 10.1085/jgp.71.4.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L. R., Cala S. E. Biochemical evidence for functional heterogeneity of cardiac sarcoplasmic reticulum vesicles. J Biol Chem. 1981 Nov 25;256(22):11809–11818. [PubMed] [Google Scholar]
- Jordan P. C. Effect of pore structure on energy barriers and applied voltage profiles. I. Symmetrical channels. Biophys J. 1984 Jun;45(6):1091–1100. doi: 10.1016/S0006-3495(84)84257-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan P. C. Effect of pore structure on energy barriers and applied voltage profiles. II. Unsymmetrical channels. Biophys J. 1984 Jun;45(6):1101–1107. doi: 10.1016/S0006-3495(84)84258-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan P. C. Electrostatic modeling of ion pores. Energy barriers and electric field profiles. Biophys J. 1982 Aug;39(2):157–164. doi: 10.1016/S0006-3495(82)84503-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch G. E., Yeh J. Z., Farley J. M., Narahashi T. Interaction of n-alkylguanidines with the sodium channels of squid axon membrane. J Gen Physiol. 1980 Sep;76(3):315–335. doi: 10.1085/jgp.76.3.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koltun W. L. Precision space-filling atomic models. Biopolymers. 1965 Dec;3(6):665–679. doi: 10.1002/bip.360030606. [DOI] [PubMed] [Google Scholar]
- Latorre R., Miller C. Conduction and selectivity in potassium channels. J Membr Biol. 1983;71(1-2):11–30. doi: 10.1007/BF01870671. [DOI] [PubMed] [Google Scholar]
- Läuger P. Diffusion-limited ion flow through pores. Biochim Biophys Acta. 1976 Dec 2;455(2):493–509. doi: 10.1016/0005-2736(76)90320-5. [DOI] [PubMed] [Google Scholar]
- Miller C. Bis-quaternary ammonium blockers as structural probes of the sarcoplasmic reticulum K+ channel. J Gen Physiol. 1982 May;79(5):869–891. doi: 10.1085/jgp.79.5.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. Coupling of water and ion fluxes in a K+-selective channel of sarcoplasmic reticulum. Biophys J. 1982 Jun;38(3):227–230. doi: 10.1016/S0006-3495(82)84552-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. Open-state substructure of single chloride channels from Torpedo electroplax. Philos Trans R Soc Lond B Biol Sci. 1982 Dec 1;299(1097):401–411. doi: 10.1098/rstb.1982.0140. [DOI] [PubMed] [Google Scholar]
- Miller C. Voltage-gated cation conductance channel from fragmented sarcoplasmic reticulum: steady-state electrical properties. J Membr Biol. 1978 Apr 20;40(1):1–23. doi: 10.1007/BF01909736. [DOI] [PubMed] [Google Scholar]
- Swenson R. P., Jr Inactivation of potassium current in squid axon by a variety of quaternary ammonium ions. J Gen Physiol. 1981 Mar;77(3):255–271. doi: 10.1085/jgp.77.3.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlins B., Williams A. J., Montgomery R. A. The characterization of a monovalent cation-selective channel of mammalian cardiac muscle sarcoplasmic reticulum. J Membr Biol. 1984;80(2):191–199. doi: 10.1007/BF01868775. [DOI] [PubMed] [Google Scholar]
- Woodhull A. M. Ionic blockage of sodium channels in nerve. J Gen Physiol. 1973 Jun;61(6):687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]


