Abstract
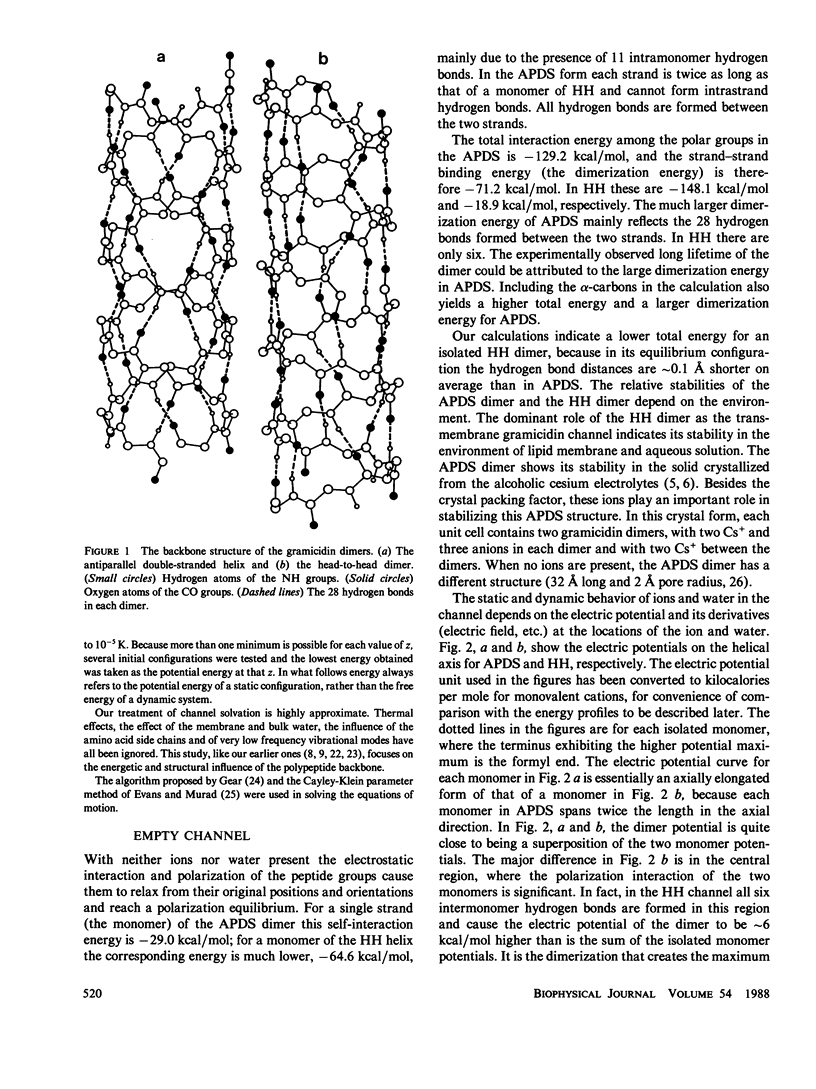
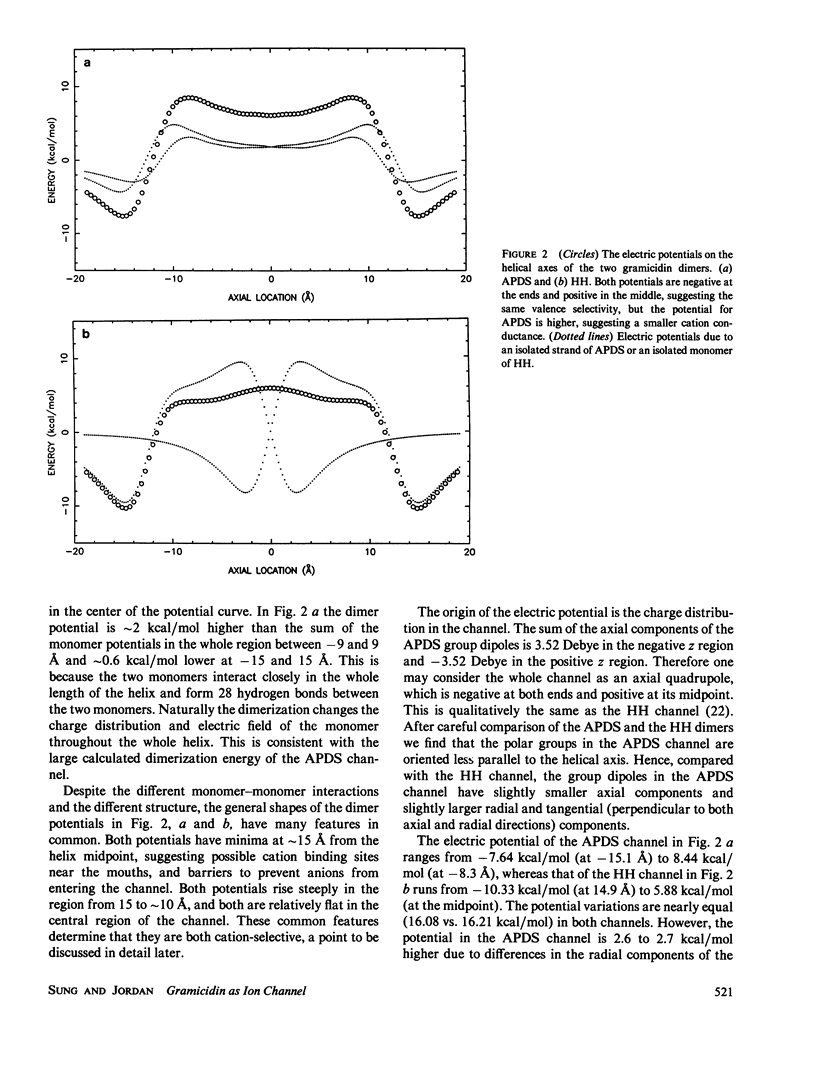
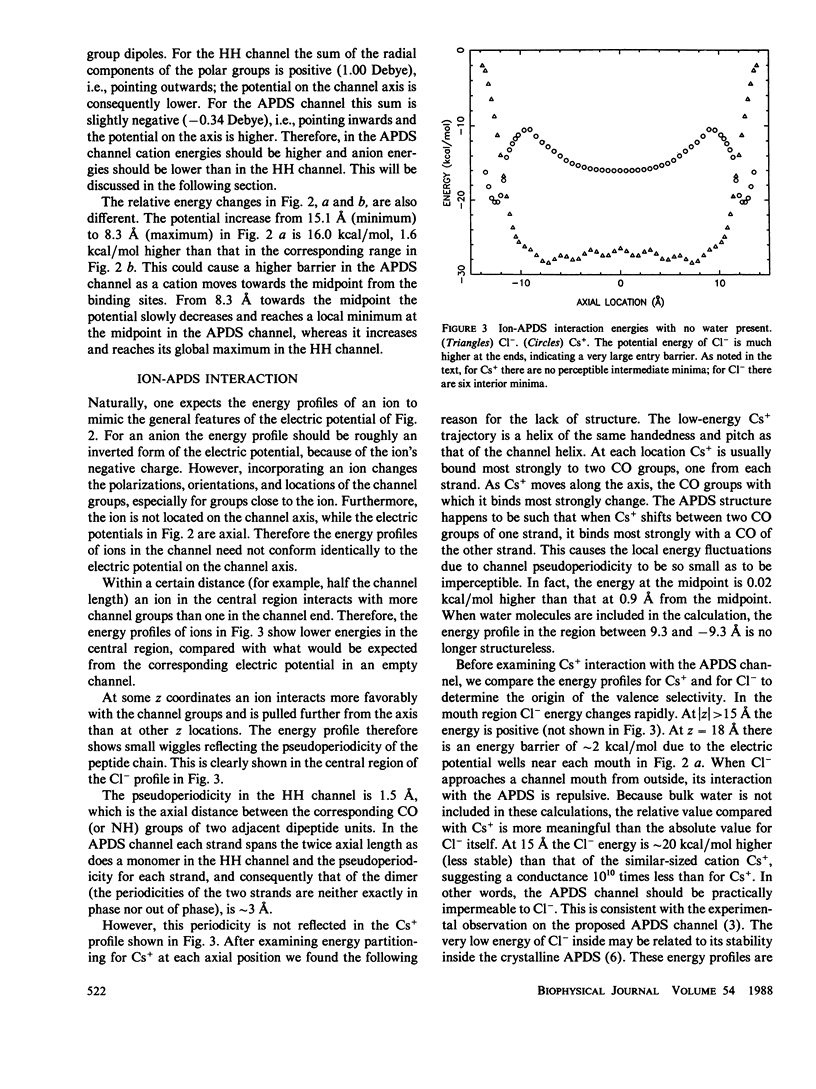
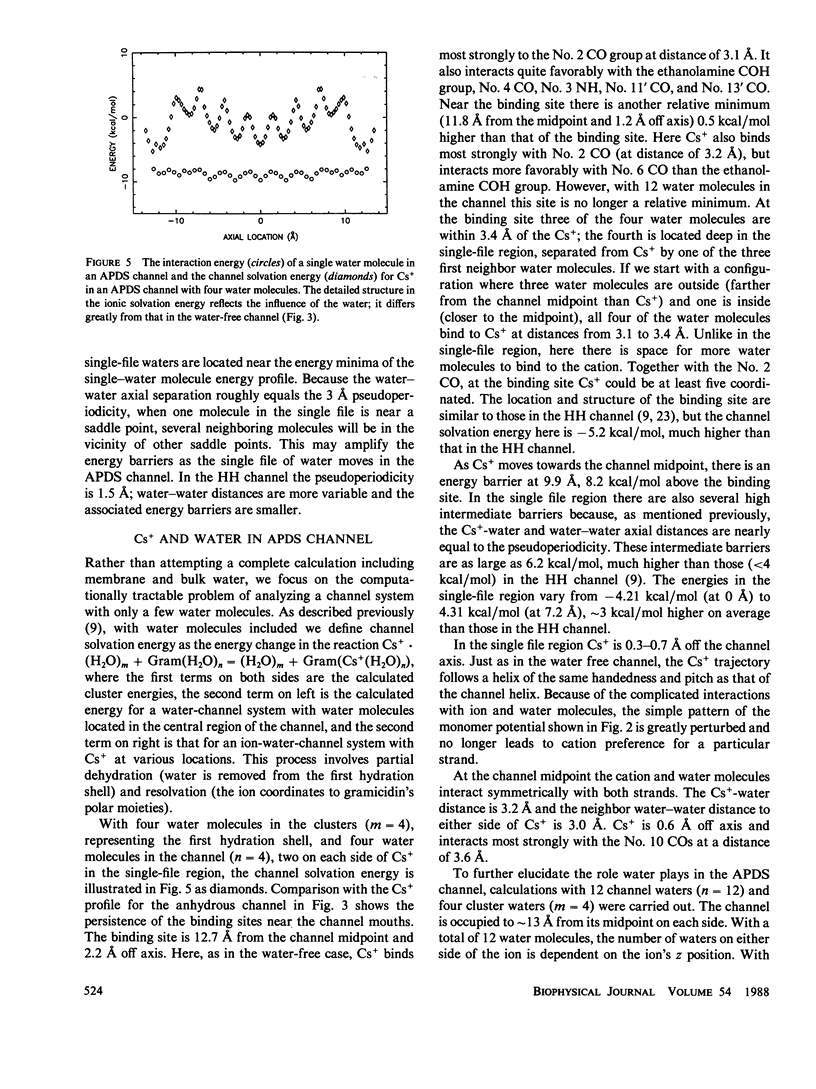
Recent experimental studies by Durkin, J. T., O. S. Andersen, F. Heitz, Y. Trudelle, and R. E. Koeppe II (1987. Biophys. J. 51:451a) have suggested that the antiparallel double-stranded helical (APDS) dimer of gramicidin can form a transmembrane cation channel. This article reports a theoretical study that successfully rationalizes the channel properties of the APDS dimer. As in the case of the head-to-head (HH) dimer, the APDS exhibits a high potential energy barrier as anions approach the channel mouth, according for the observation of valence selectivity. The calculated potential energies of cations show two binding sites near the channel mouths, a typical feature of the HH channel. The potential energies of hydrated cations in the APDS are generally higher than those in the HH channel and show a larger pseudoperiodicity and higher barriers, an observation which suggests that the APDS should exhibit lower single channel conductance.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Koeppe R. E., 2nd, Berg J. M., Hodgson K. O., Stryer L. Gramicidin A crystals contain two cation binding sites per channel. Nature. 1979 Jun 21;279(5715):723–725. doi: 10.1038/279723a0. [DOI] [PubMed] [Google Scholar]
- Koeppe R. E., 2nd, Hodgson K. O., Stryer L. Helical channels in crystals of gramicidin A and of a cesium--gramicidin A complex: an x-ray diffraction study. J Mol Biol. 1978 May 5;121(1):41–54. doi: 10.1016/0022-2836(78)90261-9. [DOI] [PubMed] [Google Scholar]
- Lee W. K., Jordan P. C. Molecular dynamics simulation of cation motion in water-filled gramicidinlike pores. Biophys J. 1984 Dec;46(6):805–819. doi: 10.1016/S0006-3495(84)84079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay D. H., Berens P. H., Wilson K. R., Hagler A. T. Structure and dynamics of ion transport through gramicidin A. Biophys J. 1984 Aug;46(2):229–248. doi: 10.1016/S0006-3495(84)84016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung S. S., Jordan P. C. The interaction of Cl- with a gramicidin-like channel. Biophys Chem. 1987 Jul;27(1):1–6. doi: 10.1016/0301-4622(87)80041-8. [DOI] [PubMed] [Google Scholar]
- Sung S. S., Jordan P. C. Why is gramicidin valence selective? A theoretical study. Biophys J. 1987 Apr;51(4):661–672. doi: 10.1016/S0006-3495(87)83391-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry D. W. The gramicidin A transmembrane channel: a proposed pi(L,D) helix. Proc Natl Acad Sci U S A. 1971 Mar;68(3):672–676. doi: 10.1073/pnas.68.3.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry D. W., Trapane T. L., Prasad K. U. Is the gramicidin a transmembrane channel single-stranded or double-stranded helix? A simple unequivocal determination. Science. 1983 Sep 9;221(4615):1064–1067. doi: 10.1126/science.221.4615.1064. [DOI] [PubMed] [Google Scholar]
- Veatch W. R., Fossel E. T., Blout E. R. The conformation of gramicidin A. Biochemistry. 1974 Dec 17;13(26):5249–5256. doi: 10.1021/bi00723a001. [DOI] [PubMed] [Google Scholar]
- Wallace B. A. Structure of gramicidin A. Biophys J. 1986 Jan;49(1):295–306. doi: 10.1016/S0006-3495(86)83642-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


