Abstract
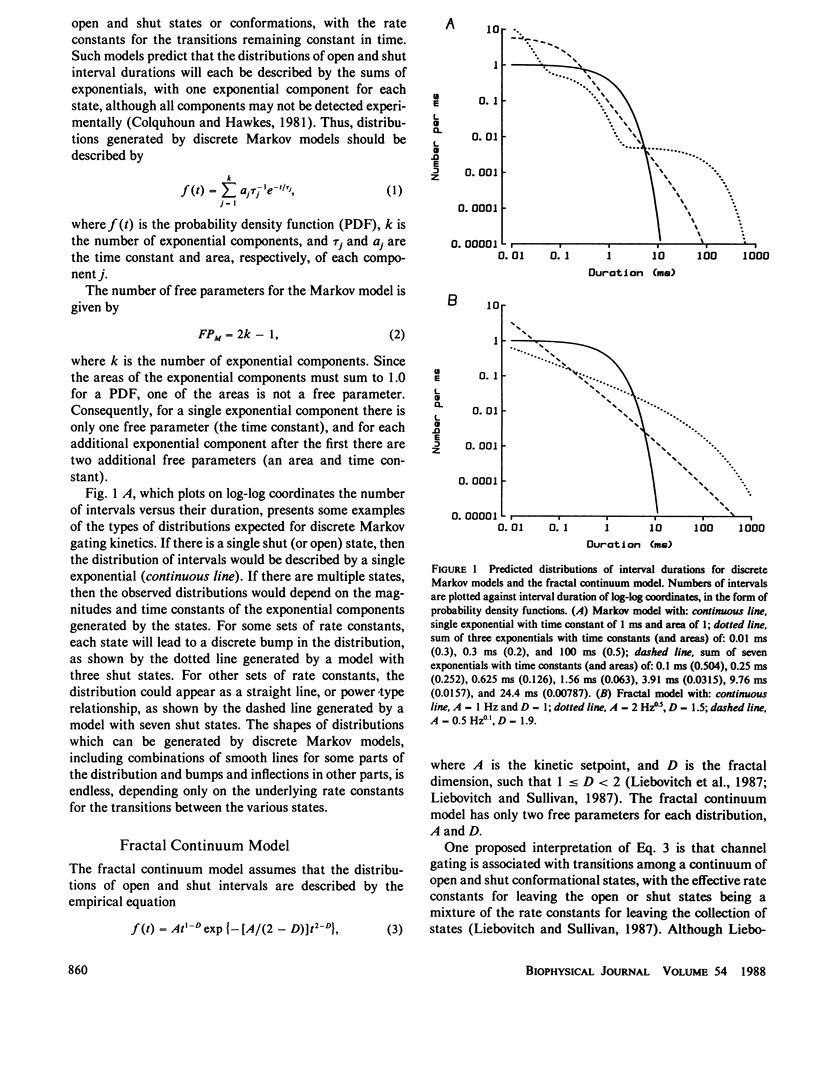
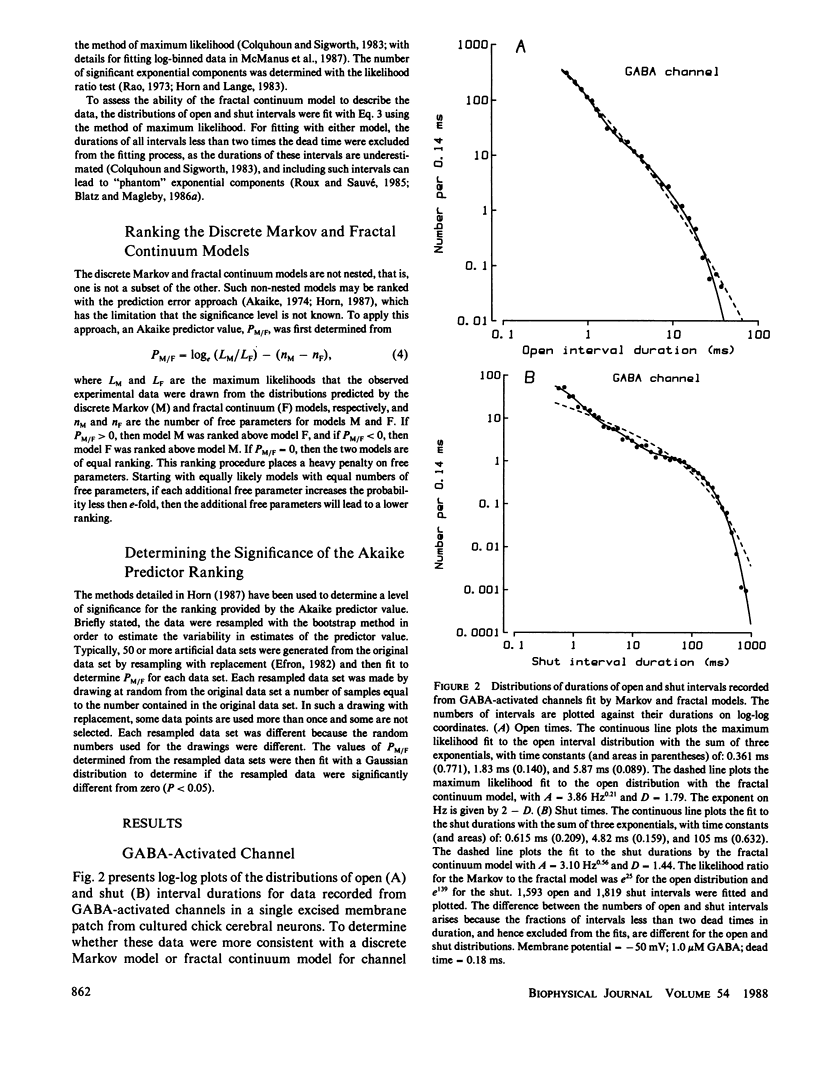
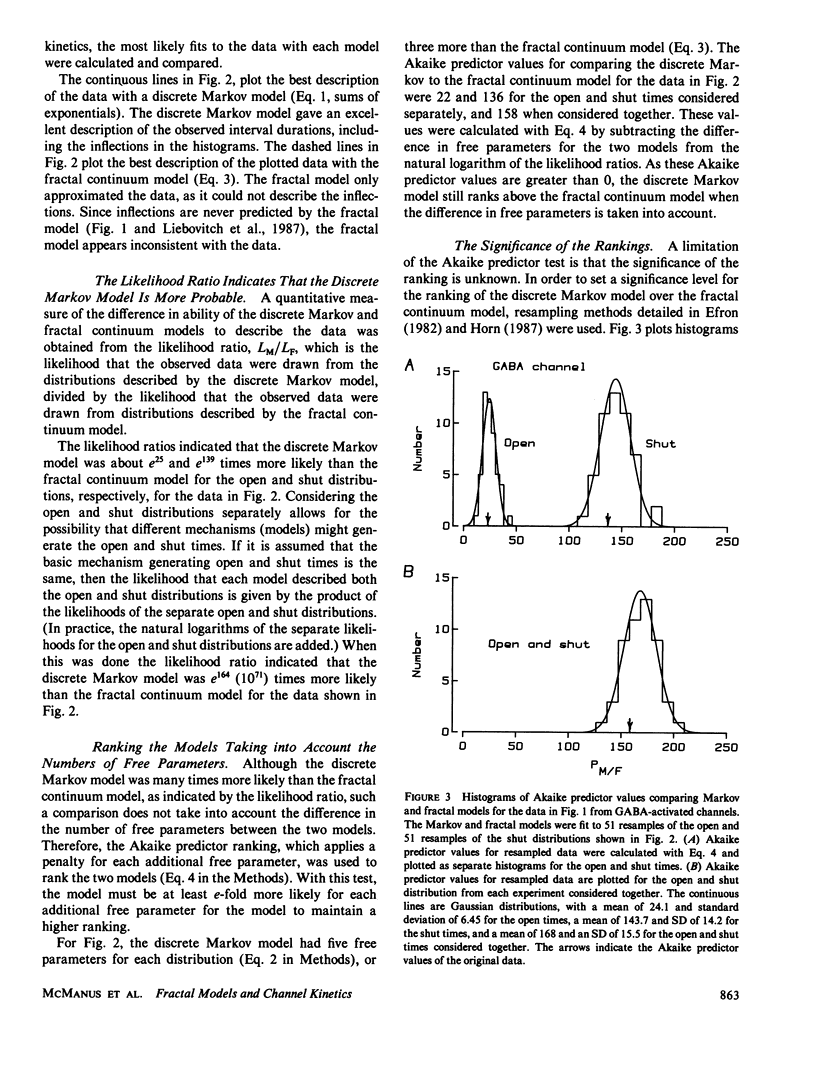
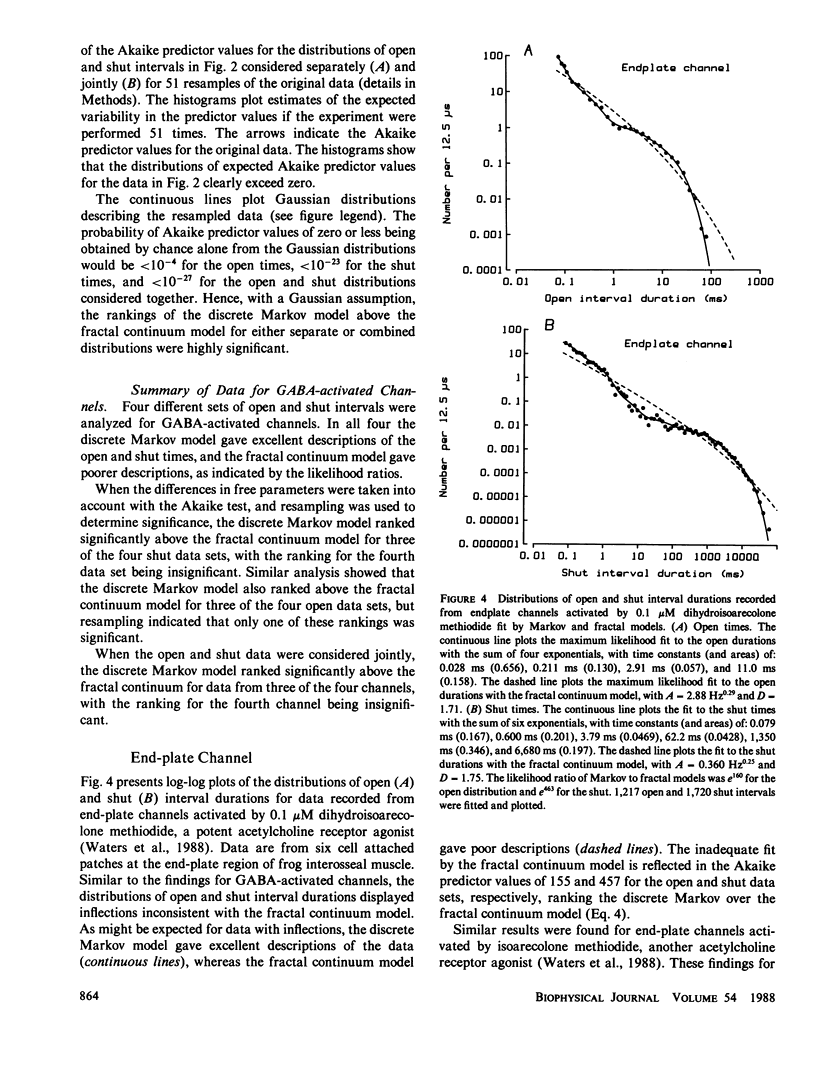
The gating kinetics of single ion channels have been well described by models which assume that channels exist in a number of discrete kinetic states, with the rate constants for transitions among the states remaining constant in time. In contrast to such discrete Markov models, it has recently been considered whether gating might arise from transitions among a continuum of states, with the effective rate constants for leaving the collections of states given by a fractal scaling equation (Liebovitch, L.S., J. Fischbarg, J.P. Koniarek, I. Todorova, and M. Wang. 1987. Biochim. Biophys. Acta. 896:173-180; Liebovitch, L.S., and J.M. Sullivan. 1987. Biophys. J. 52:979-988). The present study compares discrete Markov with fractal continuum models to determine which best describes the gating kinetics of four different ion channels: GABA-activated Cl channels, ACh-activated end-plate channels, large conductance Ca-activated K (BK) channels, and fast Cl channels. Discrete Markov models always gave excellent descriptions of the distributions of open and shut times for all four channels. Fractal continuum models typically gave very poor descriptions of the shut times for all four channels, and also of the open times from end-plate and BK channels. The descriptions of the open times from GABA-activated and fast Cl channels by the fractal and Markov models were usually not significantly different. If the same model accounts for gating motions in proteins for both the open and shut states, then the Markov model ranked above the fractal model in 35 of 36 data sets of combined open and shut intervals, with the Markov model being tens to thousands of orders of magnitude more probable. We suggest that the examined fractal continuum model is unlikely to serve as a general mechanism for the gating of these four ion channels.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldrich R. W., Corey D. P., Stevens C. F. A reinterpretation of mammalian sodium channel gating based on single channel recording. Nature. 1983 Dec 1;306(5942):436–441. doi: 10.1038/306436a0. [DOI] [PubMed] [Google Scholar]
- Ansari A., Berendzen J., Bowne S. F., Frauenfelder H., Iben I. E., Sauke T. B., Shyamsunder E., Young R. D. Protein states and proteinquakes. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5000–5004. doi: 10.1073/pnas.82.15.5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatz A. L., Magleby K. L. Correcting single channel data for missed events. Biophys J. 1986 May;49(5):967–980. doi: 10.1016/S0006-3495(86)83725-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatz A. L., Magleby K. L. Quantitative description of three modes of activity of fast chloride channels from rat skeletal muscle. J Physiol. 1986 Sep;378:141–174. doi: 10.1113/jphysiol.1986.sp016212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. On the stochastic properties of single ion channels. Proc R Soc Lond B Biol Sci. 1981 Mar 6;211(1183):205–235. doi: 10.1098/rspb.1981.0003. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Sakmann B. Fast events in single-channel currents activated by acetylcholine and its analogues at the frog muscle end-plate. J Physiol. 1985 Dec;369:501–557. doi: 10.1113/jphysiol.1985.sp015912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finer-Moore J., Stroud R. M. Amphipathic analysis and possible formation of the ion channel in an acetylcholine receptor. Proc Natl Acad Sci U S A. 1984 Jan;81(1):155–159. doi: 10.1073/pnas.81.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Horn R., Lange K. Estimating kinetic constants from single channel data. Biophys J. 1983 Aug;43(2):207–223. doi: 10.1016/S0006-3495(83)84341-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn R. Statistical methods for model discrimination. Applications to gating kinetics and permeation of the acetylcholine receptor channel. Biophys J. 1987 Feb;51(2):255–263. doi: 10.1016/S0006-3495(87)83331-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn R., Vandenberg C. A. Statistical properties of single sodium channels. J Gen Physiol. 1984 Oct;84(4):505–534. doi: 10.1085/jgp.84.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerry C. J., Ramsey R. L., Sansom M. S., Usherwood P. N. Glutamate receptor channel kinetics: the effect of glutamate concentration. Biophys J. 1988 Jan;53(1):39–52. doi: 10.1016/S0006-3495(88)83064-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn S. J., Horn R. Statistical discrimination of fractal and Markov models of single-channel gating. Biophys J. 1988 Nov;54(5):871–877. doi: 10.1016/S0006-3495(88)83023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labarca P., Rice J. A., Fredkin D. R., Montal M. Kinetic analysis of channel gating. Application to the cholinergic receptor channel and the chloride channel from Torpedo californica. Biophys J. 1985 Apr;47(4):469–478. doi: 10.1016/S0006-3495(85)83939-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebovitch L. S., Fischbarg J., Koniarek J. P., Todorova I., Wang M. Fractal model of ion-channel kinetics. Biochim Biophys Acta. 1987 Jan 26;896(2):173–180. doi: 10.1016/0005-2736(87)90177-5. [DOI] [PubMed] [Google Scholar]
- Liebovitch L. S., Sullivan J. M. Fractal analysis of a voltage-dependent potassium channel from cultured mouse hippocampal neurons. Biophys J. 1987 Dec;52(6):979–988. doi: 10.1016/S0006-3495(87)83290-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Läuger P. Internal motions in proteins and gating kinetics of ionic channels. Biophys J. 1988 Jun;53(6):877–884. doi: 10.1016/S0006-3495(88)83168-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Pallotta B. S. Calcium dependence of open and shut interval distributions from calcium-activated potassium channels in cultured rat muscle. J Physiol. 1983 Nov;344:585–604. doi: 10.1113/jphysiol.1983.sp014957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus O. B., Blatz A. L., Magleby K. L. Inverse relationship of the durations of adjacent open and shut intervals for C1 and K channels. Nature. 1985 Oct 17;317(6038):625–627. doi: 10.1038/317625a0. [DOI] [PubMed] [Google Scholar]
- McManus O. B., Blatz A. L., Magleby K. L. Sampling, log binning, fitting, and plotting durations of open and shut intervals from single channels and the effects of noise. Pflugers Arch. 1987 Nov;410(4-5):530–553. doi: 10.1007/BF00586537. [DOI] [PubMed] [Google Scholar]
- McManus O. B., Magleby K. L. Kinetic states and modes of single large-conductance calcium-activated potassium channels in cultured rat skeletal muscle. J Physiol. 1988 Aug;402:79–120. doi: 10.1113/jphysiol.1988.sp017195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. Integral membrane channels: studies in model membranes. Physiol Rev. 1983 Oct;63(4):1209–1242. doi: 10.1152/physrev.1983.63.4.1209. [DOI] [PubMed] [Google Scholar]
- Millhauser G. L., Salpeter E. E., Oswald R. E. Diffusion models of ion-channel gating and the origin of power-law distributions from single-channel recording. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1503–1507. doi: 10.1073/pnas.85.5.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moczydlowski E., Latorre R. Gating kinetics of Ca2+-activated K+ channels from rat muscle incorporated into planar lipid bilayers. Evidence for two voltage-dependent Ca2+ binding reactions. J Gen Physiol. 1983 Oct;82(4):511–542. doi: 10.1085/jgp.82.4.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Sakmann B. Single-channel currents recorded from membrane of denervated frog muscle fibres. Nature. 1976 Apr 29;260(5554):799–802. doi: 10.1038/260799a0. [DOI] [PubMed] [Google Scholar]
- Roux B., Sauvé R. A general solution to the time interval omission problem applied to single channel analysis. Biophys J. 1985 Jul;48(1):149–158. doi: 10.1016/S0006-3495(85)83768-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigworth F. J., Sine S. M. Data transformations for improved display and fitting of single-channel dwell time histograms. Biophys J. 1987 Dec;52(6):1047–1054. doi: 10.1016/S0006-3495(87)83298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sine S. M., Steinbach J. H. Activation of acetylcholine receptors on clonal mammalian BC3H-1 cells by high concentrations of agonist. J Physiol. 1987 Apr;385:325–359. doi: 10.1113/jphysiol.1987.sp016496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters J. A., Spivak C. E., Hermsmeier M., Yadav J. S., Liang R. F., Gund T. M. Synthesis, pharmacology, and molecular modeling studies of semirigid, nicotinic agonists. J Med Chem. 1988 Mar;31(3):545–554. doi: 10.1021/jm00398a010. [DOI] [PubMed] [Google Scholar]
- Weiss D. S., Barnes E. M., Jr, Hablitz J. J. Whole-cell and single-channel recordings of GABA-gated currents in cultured chick cerebral neurons. J Neurophysiol. 1988 Feb;59(2):495–513. doi: 10.1152/jn.1988.59.2.495. [DOI] [PubMed] [Google Scholar]
- Weiss D. S. Membrane potential modulates the activation of GABA-gated channels. J Neurophysiol. 1988 Feb;59(2):514–527. doi: 10.1152/jn.1988.59.2.514. [DOI] [PubMed] [Google Scholar]


