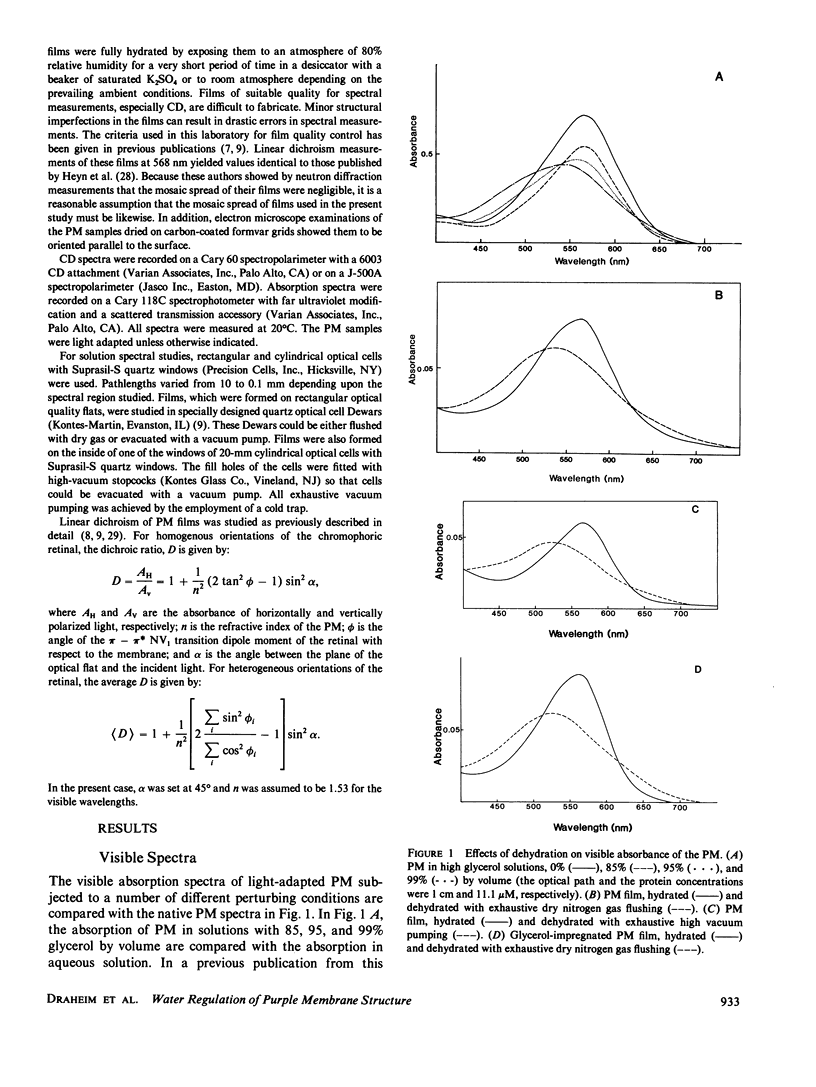
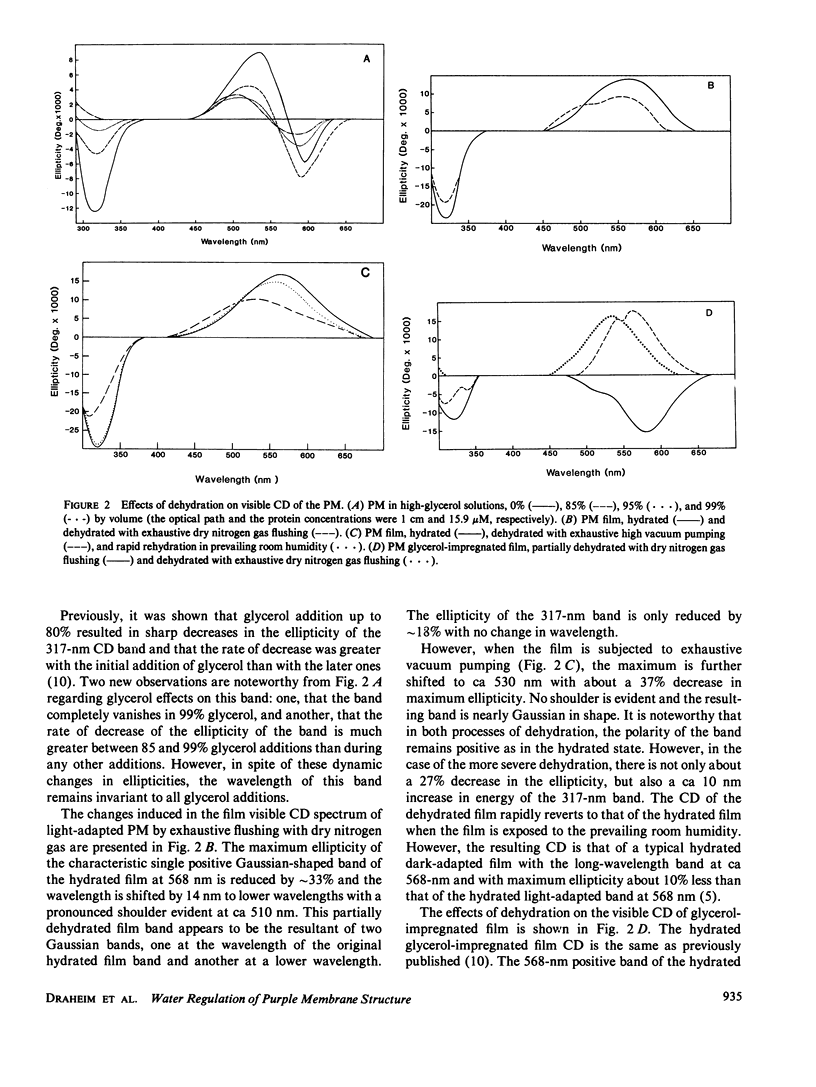
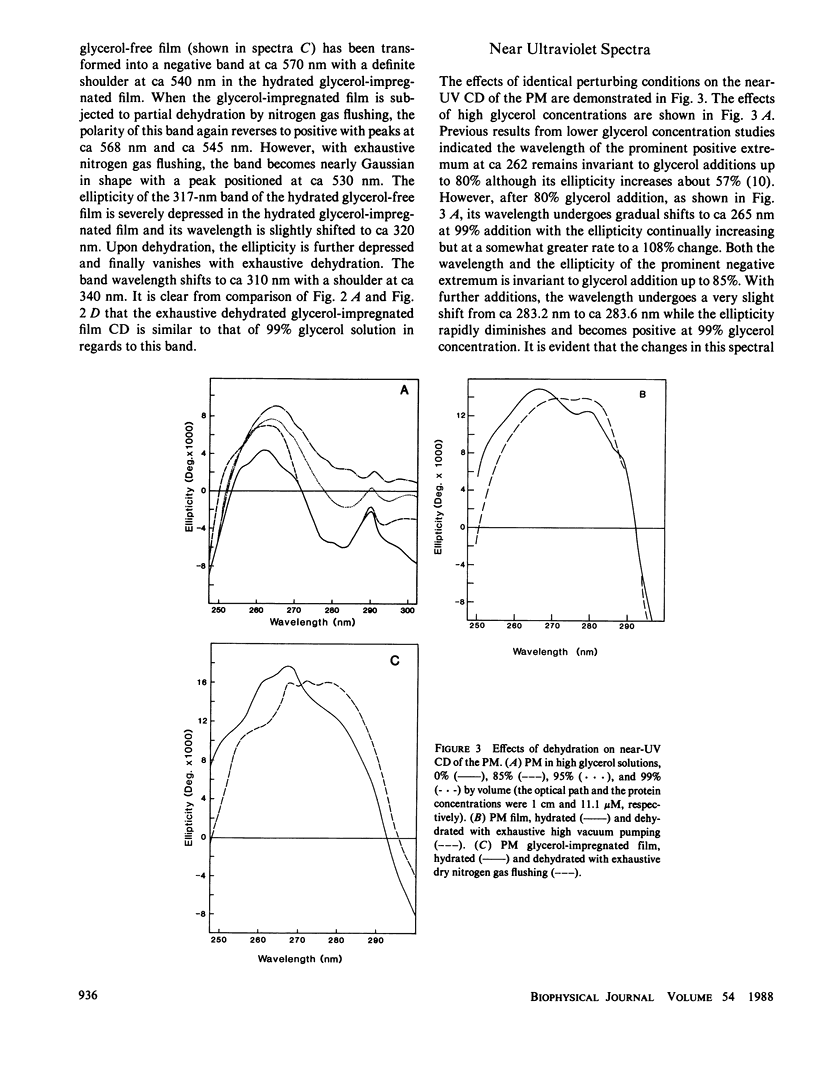
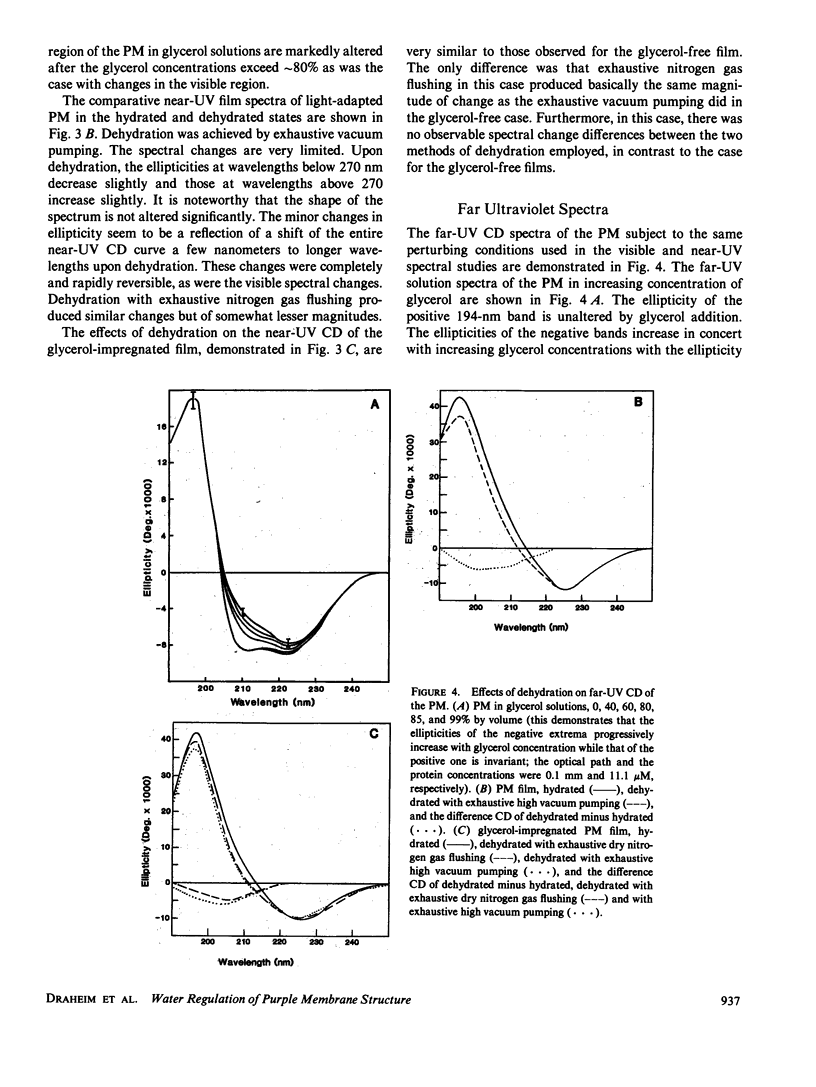
Abstract
The nature and extent of dehydration-induced molecular structural changes of the purple membrane of Halobacterium halobium have been studied by absorption and circular dichroism spectra in solution and in oriented membrane films. High glycerol concentrations, exhaustive dry nitrogen gas flushing, and exhaustive high-vacuum pumping were employed as dehydrants. The effect of these dehydrants on the spectra were reversible, similar, and additive. Analysis of the spectral changes observed at maximal dehydration revealed: (a) at least two additional optical states of the bacteriorhodopsin, one at higher energy and another at lower energy than the characteristic dark- and light-adapted states; (b) no change in the dichroic ratio at the visible absorption maximum within experimental error; (c) no change in the polarity of the visible monomeric retinylidene circular dichroic bands; (d) pronounced reduction in the characteristic excitonic interactions among the retinals in the hexagonal crystalline lattice of the membrane; (e) no changes in the native structural anisotropism of the membrane in respect to the orientation of the amino acid aromatic rings of the bacteriorhodopsin; (f) no changes in the secondary structure of the bacteriorhodopsin; and (g) a net tilting of ∼20.5° per segment of the helical polypeptide segments of the bacteriorhodopsin away from the membrane normal. A molecular model of the structural changes of the membrane resulting from water removal consistent with these findings can be constructed. Dehydration results in only subtle localized tertiary structural changes of the protein which do not significantly alter its shape or size. However, there are pronounced global supramolecular structural changes of the membrane. Water removal, which is most likely to be from the lipid headgroups of the membrane, disrupts the interactions responsible for maintaining the native crystalline lattice of the membrane resulting in pronounced randomization of the positions of the proteins in the membrane.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagley K., Dollinger G., Eisenstein L., Singh A. K., Zimányi L. Fourier transform infrared difference spectroscopy of bacteriorhodopsin and its photoproducts. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4972–4976. doi: 10.1073/pnas.79.16.4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer P. J., Dencher N. A., Heyn M. P. Evidence for chromophore-chromophore interactions in the purple membrane from reconstitution experiments of the chromophore-free membrane. Biophys Struct Mech. 1976 Apr 15;2(1):79–92. doi: 10.1007/BF00535654. [DOI] [PubMed] [Google Scholar]
- Bazzi M. D., Woody R. W. Oriented secondary structure in integral membrane proteins. I. Circular dichroism and infrared spectroscopy of cytochrome oxidase in multilamellar films. Biophys J. 1985 Dec;48(6):957–966. doi: 10.1016/S0006-3495(85)83859-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becher B. M., Cassim J. Y. Improved isolation procedures for the purple membrane of Halobacterium halobium. Prep Biochem. 1975;5(2):161–178. doi: 10.1080/00327487508061568. [DOI] [PubMed] [Google Scholar]
- Becher B., Cassim J. Y. Effects of bleaching and regeneration on the purple membrane structure of Halobaterium halobium. Biophys J. 1977 Sep;19(3):285–297. doi: 10.1016/s0006-3495(77)85588-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becher B., Cassim J. Y. Effects of light adaptation on the purple membrane structure of Halobacterium halobium. Biophys J. 1976 Oct;16(10):1183–1200. doi: 10.1016/S0006-3495(76)85767-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becher B., Ebrey T. G. Evidence for chromophore-chromophore (exciton) interaction in the purple membrane of Halobacterium halobium. Biochem Biophys Res Commun. 1976 Mar 8;69(1):1–6. doi: 10.1016/s0006-291x(76)80263-x. [DOI] [PubMed] [Google Scholar]
- Draheim J. E., Cassim J. Y. Large Scale Global Structural Changes of the Purple Membrane during the Photocycle. Biophys J. 1985 Apr;47(4):497–507. doi: 10.1016/S0006-3495(85)83943-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnest T. N., Roepe P., Braiman M. S., Gillespie J., Rothschild K. J. Orientation of the bacteriorhodopsin chromophore probed by polarized Fourier transform infrared difference spectroscopy. Biochemistry. 1986 Dec 2;25(24):7793–7798. doi: 10.1021/bi00372a002. [DOI] [PubMed] [Google Scholar]
- Ebrey T. G., Becher B., Mao B., Kilbride P., Honig B. Exciton interactions and chromophore orientation in the purple membrane. J Mol Biol. 1977 May 25;112(3):377–397. doi: 10.1016/s0022-2836(77)80188-5. [DOI] [PubMed] [Google Scholar]
- Glaeser R. M., Baldwin J., Ceska T. A., Henderson R. Electron diffraction analysis of the M412 intermediate of bacteriorhodopsin. Biophys J. 1986 Nov;50(5):913–920. doi: 10.1016/S0006-3495(86)83532-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaeser R. M., Jap B. K. Absorption flattening in the circular dichroism spectra of small membrane fragments. Biochemistry. 1985 Nov 5;24(23):6398–6401. doi: 10.1021/bi00344a012. [DOI] [PubMed] [Google Scholar]
- Glaser M., Singer S. J. Circular dichroism and the conformations of membrane proteins. Studies with red blood cell membranes. Biochemistry. 1971 May 11;10(10):1780–1787. doi: 10.1021/bi00786a008. [DOI] [PubMed] [Google Scholar]
- Gordon D. J., Holzwarth G. Optical activity of membrane suspensions: calculation of artifacts by Mie scattering theory. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2365–2369. doi: 10.1073/pnas.68.10.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon D. Discussion paper: classical scattering calculation of particulate artifacts in membrane optical activity. Ann N Y Acad Sci. 1972 Jun 20;195:147–149. [PubMed] [Google Scholar]
- Henderson R. The purple membrane from Halobacterium halobium. Annu Rev Biophys Bioeng. 1977;6:87–109. doi: 10.1146/annurev.bb.06.060177.000511. [DOI] [PubMed] [Google Scholar]
- Henderson R. The structure of the purple membrane from Halobacterium hallobium: analysis of the X-ray diffraction pattern. J Mol Biol. 1975 Apr 5;93(2):123–138. doi: 10.1016/0022-2836(75)90123-0. [DOI] [PubMed] [Google Scholar]
- Henderson R., Unwin P. N. Three-dimensional model of purple membrane obtained by electron microscopy. Nature. 1975 Sep 4;257(5521):28–32. doi: 10.1038/257028a0. [DOI] [PubMed] [Google Scholar]
- Heyn M. P., Bauer P. J., Dencher N. A. A natural CD label to probe the structure of the purple membrane from Halobacterium halobium by means of exciton coupling effects. Biochem Biophys Res Commun. 1975 Dec 1;67(3):897–903. doi: 10.1016/0006-291x(75)90761-5. [DOI] [PubMed] [Google Scholar]
- Heyn M. P., Cherry R. J., Müller U. Transient and linear dichroism studies on bacteriorhodopsin: determination of the orientation of the 568 nm all-trans retinal chromophore. J Mol Biol. 1977 Dec 15;117(3):607–620. doi: 10.1016/0022-2836(77)90060-2. [DOI] [PubMed] [Google Scholar]
- Hwang S. B., Korenbrot J. I., Stoeckenius W. Structural and spectroscopic characteristics of bacteriorhodopsin in air-water interface films. J Membr Biol. 1977 Sep 14;36(2-3):115–135. doi: 10.1007/BF01868147. [DOI] [PubMed] [Google Scholar]
- Jap B. K., Maestre M. F., Hayward S. B., Glaeser R. M. Peptide-chain secondary structure of bacteriorhodopsin. Biophys J. 1983 Jul;43(1):81–89. doi: 10.1016/S0006-3495(83)84326-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisky O., Ottolenghi M., Honig B., Korenstein R. Environmental effects on formation and photoreaction of the M412 photoproduct of bacteriorhodopsin: implications for the mechanism of proton pumping. Biochemistry. 1981 Feb 3;20(3):649–655. doi: 10.1021/bi00506a031. [DOI] [PubMed] [Google Scholar]
- Korenstein R., Hess B. Hydration effects on cis--trans isomerization of bacteriorhodopsin. FEBS Lett. 1977 Oct 1;82(1):7–11. doi: 10.1016/0014-5793(77)80874-0. [DOI] [PubMed] [Google Scholar]
- Korenstein R., Hess B. Hydration effects on the photocycle of bacteriorhodopsin in thin layers of purple membrane. Nature. 1977 Nov 10;270(5633):184–186. doi: 10.1038/270184a0. [DOI] [PubMed] [Google Scholar]
- Krimm S., Dwivedi A. M. Infrared spectrum of the purple membrane: clue to a proton conduction mechanism? Science. 1982 Apr 23;216(4544):407–408. doi: 10.1126/science.6280277. [DOI] [PubMed] [Google Scholar]
- Lazarev Y. A., Terpugov E. L. Effect of water on the structure of bacteriorhodopsin and photochemical processes in purple membranes. Biochim Biophys Acta. 1980 May 9;590(3):324–338. doi: 10.1016/0005-2728(80)90203-0. [DOI] [PubMed] [Google Scholar]
- Mao D., Wallace B. A. Differential light scattering and absorption flattening optical effects are minimal in the circular dichroism spectra of small unilamellar vesicles. Biochemistry. 1984 Jun 5;23(12):2667–2673. doi: 10.1021/bi00307a020. [DOI] [PubMed] [Google Scholar]
- Muccio D. D., Cassim J. Y. Interpretation of the absorption and circular dichroic spectra of oriented purple membrane films. Biophys J. 1979 Jun;26(3):427–440. doi: 10.1016/S0006-3495(79)85263-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muccio D. D., Cassim J. Y. Interpretations of the effects of pH on the spectra of purple membrane. J Mol Biol. 1979 Dec 15;135(3):595–609. doi: 10.1016/0022-2836(79)90166-9. [DOI] [PubMed] [Google Scholar]
- Nabedryk E., Bardin A. M., Breton J. Further characterization of protein secondary structures in purple membrane by circular dichroism and polarized infrared spectroscopies. Biophys J. 1985 Dec;48(6):873–876. doi: 10.1016/S0006-3495(85)83848-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagle J. F., Parodi L. A., Lozier R. H. Procedure for testing kinetic models of the photocycle of bacteriorhodopsin. Biophys J. 1982 May;38(2):161–174. doi: 10.1016/S0006-3495(82)84543-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy K. Photoelectric activity of dried, oriented layers of purple membrane from Halobacterium halobium. Biochem Biophys Res Commun. 1978 Nov 14;85(1):383–390. doi: 10.1016/s0006-291x(78)80054-0. [DOI] [PubMed] [Google Scholar]
- Némethy G., Phillips D. C., Leach S. J., Scheraga H. A. A second right-handed helical structure with the parameters of the Pauling-Corey alpha-helix. Nature. 1967 Apr 22;214(5086):363–365. doi: 10.1038/214363a0. [DOI] [PubMed] [Google Scholar]
- Ottaway C. A., Wetlaufer D. B. Light-scattering contributions to the circular dichroism of particulate systems. Arch Biochem Biophys. 1970 Aug;139(2):257–264. doi: 10.1016/0003-9861(70)90476-5. [DOI] [PubMed] [Google Scholar]
- Papadopoulos G. K., Cassim J. Y. Orientations of the retinyl and the heme chromophores in the brown membrane of Halobacterium halobium. J Mol Biol. 1981 Oct 15;152(1):35–47. doi: 10.1016/0022-2836(81)90094-2. [DOI] [PubMed] [Google Scholar]
- Rothschild K. J., Clark N. A. Polarized infrared spectroscopy of oriented purple membrane. Biophys J. 1979 Mar;25(3):473–487. doi: 10.1016/S0006-3495(79)85317-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider A. S., Schneider M. J., Rosenheck K. Optical activity of biological membranes: scattering effects and protein conformation. Proc Natl Acad Sci U S A. 1970 Jul;66(3):793–798. doi: 10.1073/pnas.66.3.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckenius W., Bogomolni R. A. Bacteriorhodopsin and related pigments of halobacteria. Annu Rev Biochem. 1982;51:587–616. doi: 10.1146/annurev.bi.51.070182.003103. [DOI] [PubMed] [Google Scholar]
- Stoeckenius W., Lozier R. H., Bogomolni R. A. Bacteriorhodopsin and the purple membrane of halobacteria. Biochim Biophys Acta. 1979 Mar 14;505(3-4):215–278. doi: 10.1016/0304-4173(79)90006-5. [DOI] [PubMed] [Google Scholar]
- Urry D. W., Ji T. H. Distortions in circular dichroism patterns of particulate (or membranous) systems. Arch Biochem Biophys. 1968 Dec;128(3):802–807. doi: 10.1016/0003-9861(68)90088-x. [DOI] [PubMed] [Google Scholar]
- Váró G., Eisenstein L. Infrared studies of water induced conformational changes in bacteriorhodopsin. Eur Biophys J. 1987;14(3):163–168. doi: 10.1007/BF00253841. [DOI] [PubMed] [Google Scholar]
- Váró G., Keszthelyi L. Photoelectric signals from dried oriented purple membranes of Halobacterium halobium. Biophys J. 1983 Jul;43(1):47–51. doi: 10.1016/S0006-3495(83)84322-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace B. A., Teeters C. L. Differential absorption flattening optical effects are significant in the circular dichroism spectra of large membrane fragments. Biochemistry. 1987 Jan 13;26(1):65–70. doi: 10.1021/bi00375a010. [DOI] [PubMed] [Google Scholar]
- Woody R. W. Improved calculation of the n-pi rotational strength in polypeptides. J Chem Phys. 1968 Dec 1;49(11):4797–4806. doi: 10.1063/1.1669962. [DOI] [PubMed] [Google Scholar]
- Zaccai G., Gilmore D. J. Areas of hydration in the purple membrane of Halobacterium halobium: a neutron diffraction study. J Mol Biol. 1979 Aug 5;132(2):181–191. doi: 10.1016/0022-2836(79)90390-5. [DOI] [PubMed] [Google Scholar]
- Zaccai G. Structure and hydration of purple membranes in different conditions. J Mol Biol. 1987 Apr 5;194(3):569–572. doi: 10.1016/0022-2836(87)90683-8. [DOI] [PubMed] [Google Scholar]


