Abstract
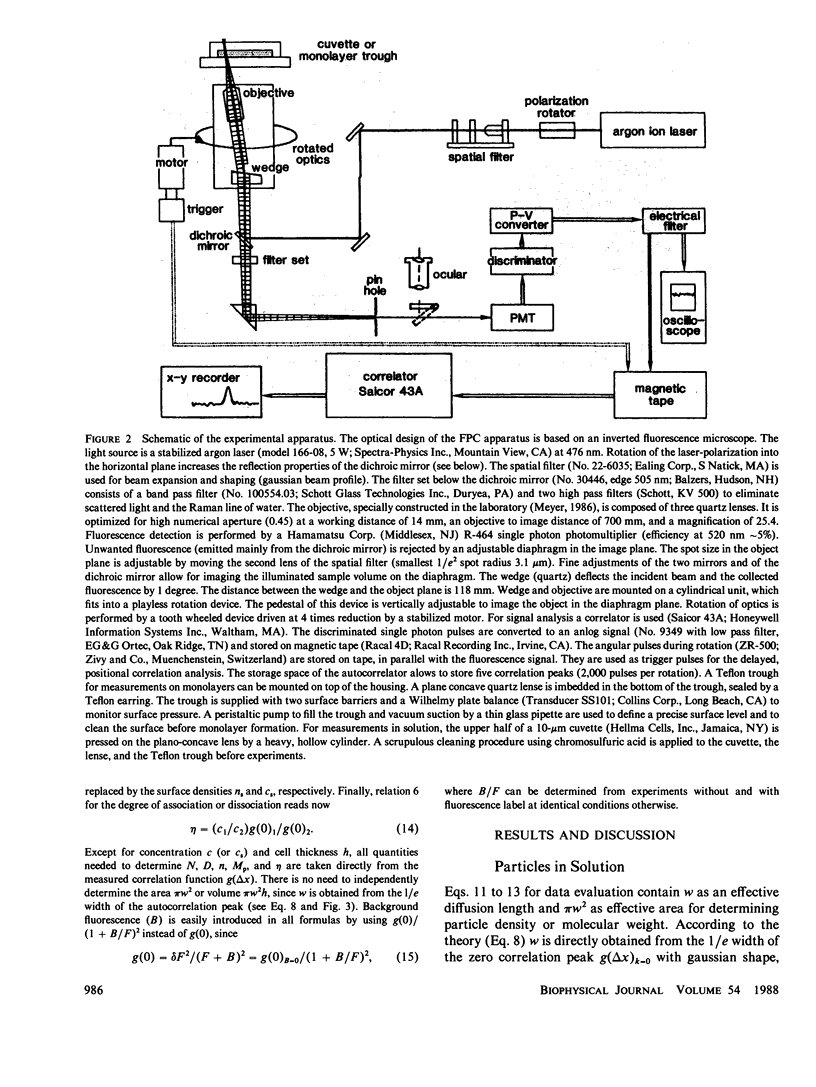
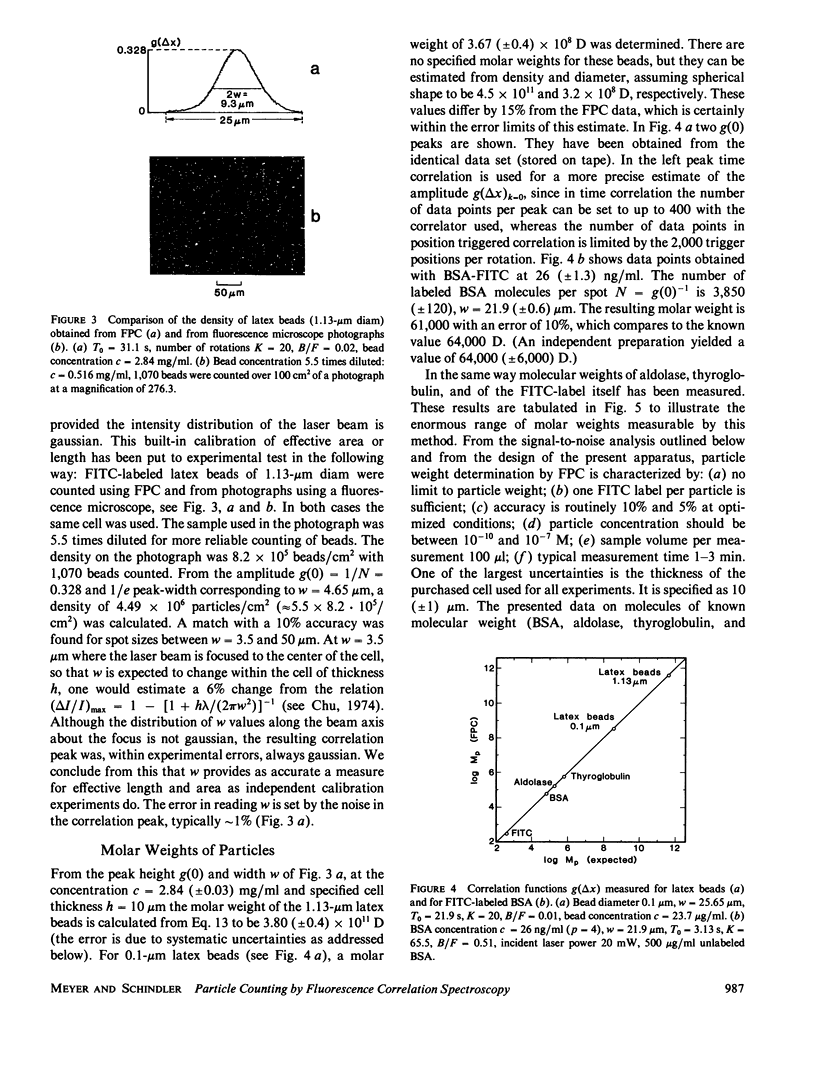
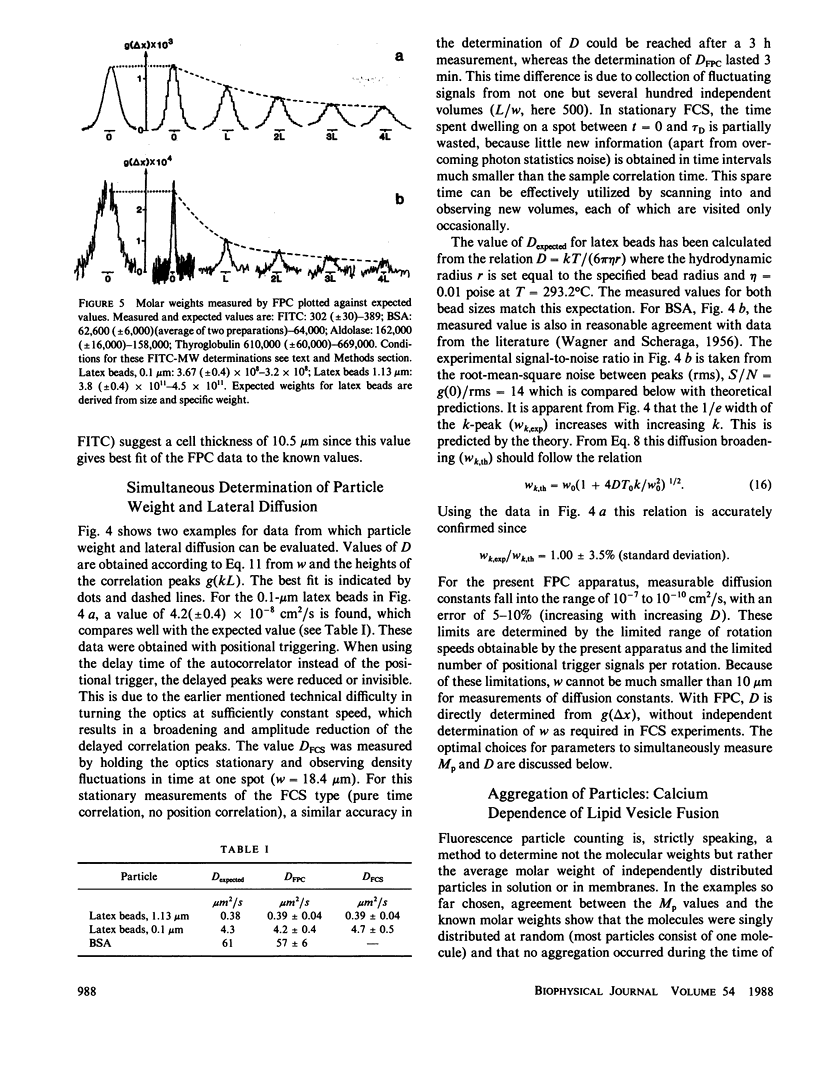
A method for simultaneous determination of molar weights (M) and lateral diffusion constants (D) of particles in three- and two-dimensional systems is described. Spontaneous concentration fluctuations in space and time are analyzed, by monitoring fluctuations in the fluorescence from fluorescein-labeled molecules (1 dye/molecule is sufficient), excited by a rotating laser spot. For particles in solution, M values are determined over the range of 3 x 10(2) to 3 x 10(11) daltons, and D values can be determined from approximately 10(-7) to 10(-10) cm2/s. The time for a determination is approximately 1 min. Aggregation can be followed by changes of either M or D. This method is used to study the calcium dependence of vesicle aggregation or fusion, and the time course of aggregate formation of porin (an Escherichia Coli outer membrane protein) in lipid monolayers. Essential parameters for the development of the method are described. Equations to estimate the signal-to-noise ratios and to find the optimal free parameters for a specific application are derived. The theoretical predictions for the correlation function of the signal and for the signal-to-noise ratio are compared with observed values.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrod D., Thompson N. L., Burghardt T. P. Total internal inflection fluorescent microscopy. J Microsc. 1983 Jan;129(Pt 1):19–28. doi: 10.1111/j.1365-2818.1983.tb04158.x. [DOI] [PubMed] [Google Scholar]
- Cherry R. J. Rotational and lateral diffusion of membrane proteins. Biochim Biophys Acta. 1979 Dec 20;559(4):289–327. doi: 10.1016/0304-4157(79)90009-1. [DOI] [PubMed] [Google Scholar]
- Day E. P., Ho J. T., Kunze R. K., Jr, Sun S. T. Dynamic light scattering study of calcium-induced fusion in phospholipid vesicles. Biochim Biophys Acta. 1977 Nov 1;470(3):503–508. doi: 10.1016/0005-2736(77)90142-0. [DOI] [PubMed] [Google Scholar]
- Düzgünes N., Nir S., Wilschut J., Bentz J., Newton C., Portis A., Papahadjopoulos D. Calcium- and magnesium-induced fusion of mixed phosphatidylserine/phosphatidylcholine vesicles: effect of ion binding. J Membr Biol. 1981 Apr 15;59(2):115–125. doi: 10.1007/BF01875709. [DOI] [PubMed] [Google Scholar]
- Elson E. L., Webb W. W. Concentration correlation spectroscopy: a new biophysical probe based on occupation number fluctuations. Annu Rev Biophys Bioeng. 1975;4(00):311–334. doi: 10.1146/annurev.bb.04.060175.001523. [DOI] [PubMed] [Google Scholar]
- Engel A., Massalski A., Schindler H., Dorset D. L., Rosenbusch J. P. Porin channel triplets merge into single outlets in Escherichia coli outer membranes. Nature. 1985 Oct 17;317(6038):643–645. doi: 10.1038/317643a0. [DOI] [PubMed] [Google Scholar]
- Garavito R. M., Rosenbusch J. P. Three-dimensional crystals of an integral membrane protein: an initial x-ray analysis. J Cell Biol. 1980 Jul;86(1):327–329. doi: 10.1083/jcb.86.1.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross D., Webb W. W. Molecular counting of low-density lipoprotein particles as individuals and small clusters on cell surfaces. Biophys J. 1986 Apr;49(4):901–911. doi: 10.1016/S0006-3495(86)83718-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Icenogle R. D., Elson E. L. Fluorescence correlation spectroscopy and photobleaching recovery of multiple binding reactions. I. Theory and FCS measurements. Biopolymers. 1983 Aug;22(8):1919–1948. doi: 10.1002/bip.360220808. [DOI] [PubMed] [Google Scholar]
- Kachar B., Fuller N., Rand R. P. Morphological responses to calcium-induced interaction of phosphatidylserine-containing vesicles. Biophys J. 1986 Nov;50(5):779–788. doi: 10.1016/S0006-3495(86)83518-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppel D. E., Axelrod D., Schlessinger J., Elson E. L., Webb W. W. Dynamics of fluorescence marker concentration as a probe of mobility. Biophys J. 1976 Nov;16(11):1315–1329. doi: 10.1016/S0006-3495(76)85776-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magde D., Elson E. L., Webb W. W. Fluorescence correlation spectroscopy. II. An experimental realization. Biopolymers. 1974 Jan;13(1):29–61. doi: 10.1002/bip.1974.360130103. [DOI] [PubMed] [Google Scholar]
- Nicoli D. F., Briggs J., Elings V. B. Fluorescence immunoassay based on long time correlations of number fluctuations. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4904–4908. doi: 10.1073/pnas.77.8.4904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer A. G., 3rd, Thompson N. L. Molecular aggregation characterized by high order autocorrelation in fluorescence correlation spectroscopy. Biophys J. 1987 Aug;52(2):257–270. doi: 10.1016/S0006-3495(87)83213-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer A. G., 3rd, Thompson N. L. Theory of sample translation in fluorescence correlation spectroscopy. Biophys J. 1987 Feb;51(2):339–343. doi: 10.1016/S0006-3495(87)83340-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters R., Peters J., Tews K. H., Bähr W. A microfluorimetric study of translational diffusion in erythrocyte membranes. Biochim Biophys Acta. 1974 Nov 15;367(3):282–294. doi: 10.1016/0005-2736(74)90085-6. [DOI] [PubMed] [Google Scholar]
- Petersen N. O. Diffusion and aggregation in biological membranes. Can J Biochem Cell Biol. 1984 Nov;62(11):1158–1166. doi: 10.1139/o84-149. [DOI] [PubMed] [Google Scholar]
- Petersen N. O., Elson E. L. Measurements of diffusion and chemical kinetics by fluorescence photobleaching recovery and fluorescence correlation spectroscopy. Methods Enzymol. 1986;130:454–484. doi: 10.1016/0076-6879(86)30021-1. [DOI] [PubMed] [Google Scholar]
- Petersen N. O., Johnson D. C., Schlesinger M. J. Scanning fluorescence correlation spectroscopy. II. Application to virus glycoprotein aggregation. Biophys J. 1986 Apr;49(4):817–820. doi: 10.1016/S0006-3495(86)83710-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen N. O. Scanning fluorescence correlation spectroscopy. I. Theory and simulation of aggregation measurements. Biophys J. 1986 Apr;49(4):809–815. doi: 10.1016/S0006-3495(86)83709-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler H., Rosenbusch J. P. Matrix protein from Escherichia coli outer membranes forms voltage-controlled channels in lipid bilayers. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3751–3755. doi: 10.1073/pnas.75.8.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler H., Rosenbusch J. P. Matrix protein in planar membranes: clusters of channels in a native environment and their functional reassembly. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2302–2306. doi: 10.1073/pnas.78.4.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman M., Schindler H., Feher G. Determination of molecular weights by fluctuation spectroscopy: application to DNA. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2776–2780. doi: 10.1073/pnas.73.8.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilschut J., Düzgüneş N., Papahadjopoulos D. Calcium/magnesium specificity in membrane fusion: kinetics of aggregation and fusion of phosphatidylserine vesicles and the role of bilayer curvature. Biochemistry. 1981 May 26;20(11):3126–3133. doi: 10.1021/bi00514a022. [DOI] [PubMed] [Google Scholar]


