Abstract
The bacterial flagellar motor is a molecular engine that couples the flow of protons across the cytoplasmic membrane to rotation of the flagellar filament. We analyze the steady-state behavior of an explicit mechanical model in which a fixed number of protons carries the filament through one revolution. Predictions of this model are compared with experimentally determined relationships between protonmotive force, proton flux, torque, and speed. All such tightly coupled mechanisms produce the same torque when the motor is stalled but vary greatly in their behavior at high speed. The speed at zero load predicted by our model is limited by the rates of association and dissociation of protons at binding sites on the rotor and by the mobility of force generators containing transmembrane channels that interact with these sites. Our analysis suggests that more could be learned about the motor if it were driven by an externally applied torque backwards (at negative speed) or forwards at speeds greater than the zero-load speed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg H. C., Anderson R. A. Bacteria swim by rotating their flagellar filaments. Nature. 1973 Oct 19;245(5425):380–382. doi: 10.1038/245380a0. [DOI] [PubMed] [Google Scholar]
- Berg H. C. Dynamic properties of bacterial flagellar motors. Nature. 1974 May 3;249(452):77–79. doi: 10.1038/249077a0. [DOI] [PubMed] [Google Scholar]
- Berg H. C., Manson M. D., Conley M. P. Dynamics and energetics of flagellar rotation in bacteria. Symp Soc Exp Biol. 1982;35:1–31. [PubMed] [Google Scholar]
- Blair D. F., Berg H. C. Restoration of torque in defective flagellar motors. Science. 1988 Dec 23;242(4886):1678–1681. doi: 10.1126/science.2849208. [DOI] [PubMed] [Google Scholar]
- Block S. M., Berg H. C. Successive incorporation of force-generating units in the bacterial rotary motor. 1984 May 31-Jun 6Nature. 309(5967):470–472. doi: 10.1038/309470a0. [DOI] [PubMed] [Google Scholar]
- Conley M. P., Berg H. C. Chemical modification of Streptococcus flagellar motors. J Bacteriol. 1984 Jun;158(3):832–843. doi: 10.1128/jb.158.3.832-843.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulton J. W., Murray R. G. Cell envelope associations of Aquaspirillum serpens flagella. J Bacteriol. 1978 Dec;136(3):1037–1049. doi: 10.1128/jb.136.3.1037-1049.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePamphilis M. L., Adler J. Attachment of flagellar basal bodies to the cell envelope: specific attachment to the outer, lipopolysaccharide membrane and the cyoplasmic membrane. J Bacteriol. 1971 Jan;105(1):396–407. doi: 10.1128/jb.105.1.396-407.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya R., Hotani H., Asakura S. Polymorphic transition in bacterial flagella. Symp Soc Exp Biol. 1982;35:53–76. [PubMed] [Google Scholar]
- Kashket E. R. The proton motive force in bacteria: a critical assessment of methods. Annu Rev Microbiol. 1985;39:219–242. doi: 10.1146/annurev.mi.39.100185.001251. [DOI] [PubMed] [Google Scholar]
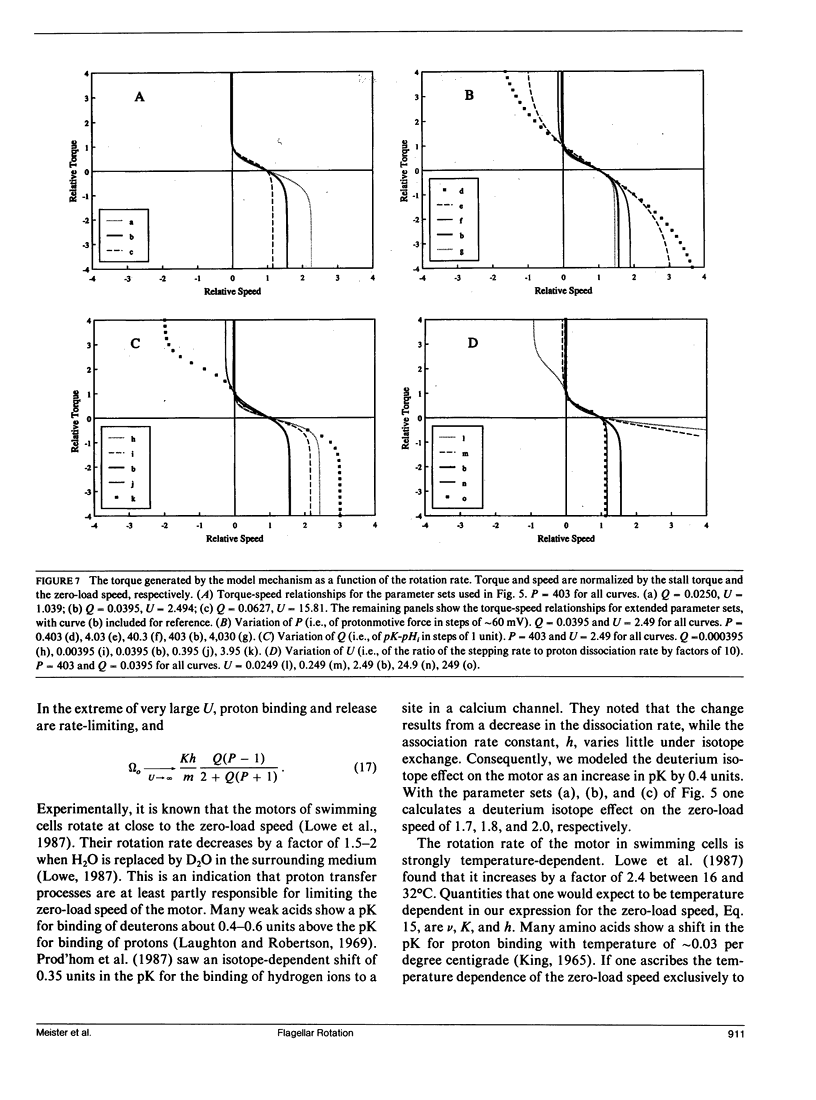
- Khan S., Berg H. C. Isotope and thermal effects in chemiosmotic coupling to the flagellar motor of Streptococcus. Cell. 1983 Mar;32(3):913–919. doi: 10.1016/0092-8674(83)90076-4. [DOI] [PubMed] [Google Scholar]
- Khan S., Dapice M., Reese T. S. Effects of mot gene expression on the structure of the flagellar motor. J Mol Biol. 1988 Aug 5;202(3):575–584. doi: 10.1016/0022-2836(88)90287-2. [DOI] [PubMed] [Google Scholar]
- Khan S., Meister M., Berg H. C. Constraints on flagellar rotation. J Mol Biol. 1985 Aug 20;184(4):645–656. doi: 10.1016/0022-2836(85)90310-9. [DOI] [PubMed] [Google Scholar]
- Läuger P. Torque and rotation rate of the bacterial flagellar motor. Biophys J. 1988 Jan;53(1):53–65. doi: 10.1016/S0006-3495(88)83065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manson M. D., Tedesco P. M., Berg H. C. Energetics of flagellar rotation in bacteria. J Mol Biol. 1980 Apr 15;138(3):541–561. doi: 10.1016/s0022-2836(80)80017-9. [DOI] [PubMed] [Google Scholar]
- Meister M., Berg H. C. The stall torque of the bacterial flagellar motor. Biophys J. 1987 Sep;52(3):413–419. doi: 10.1016/S0006-3495(87)83230-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister M., Lowe G., Berg H. C. The proton flux through the bacterial flagellar motor. Cell. 1987 Jun 5;49(5):643–650. doi: 10.1016/0092-8674(87)90540-x. [DOI] [PubMed] [Google Scholar]
- Prod'hom B., Pietrobon D., Hess P. Direct measurement of proton transfer rates to a group controlling the dihydropyridine-sensitive Ca2+ channel. Nature. 1987 Sep 17;329(6136):243–246. doi: 10.1038/329243a0. [DOI] [PubMed] [Google Scholar]
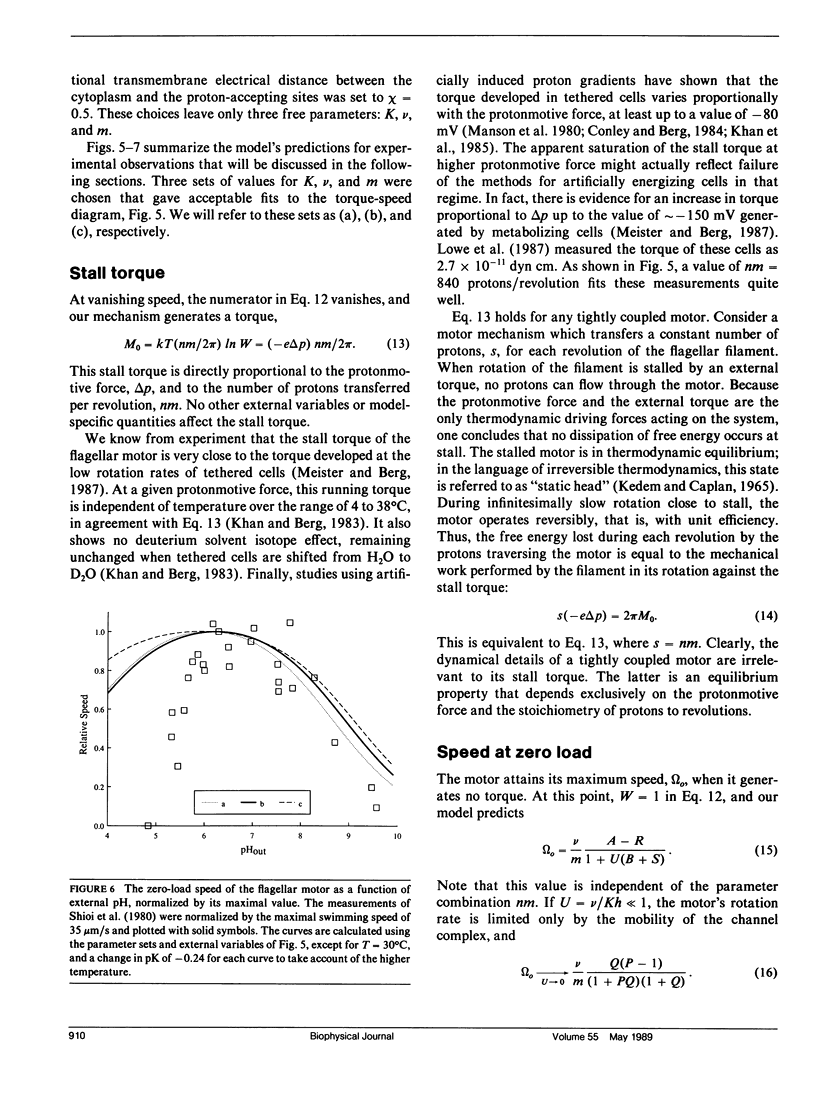
- Shioi J. I., Matsuura S., Imae Y. Quantitative measurements of proton motive force and motility in Bacillus subtilis. J Bacteriol. 1980 Dec;144(3):891–897. doi: 10.1128/jb.144.3.891-897.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman M., Simon M. Flagellar rotation and the mechanism of bacterial motility. Nature. 1974 May 3;249(452):73–74. doi: 10.1038/249073a0. [DOI] [PubMed] [Google Scholar]


