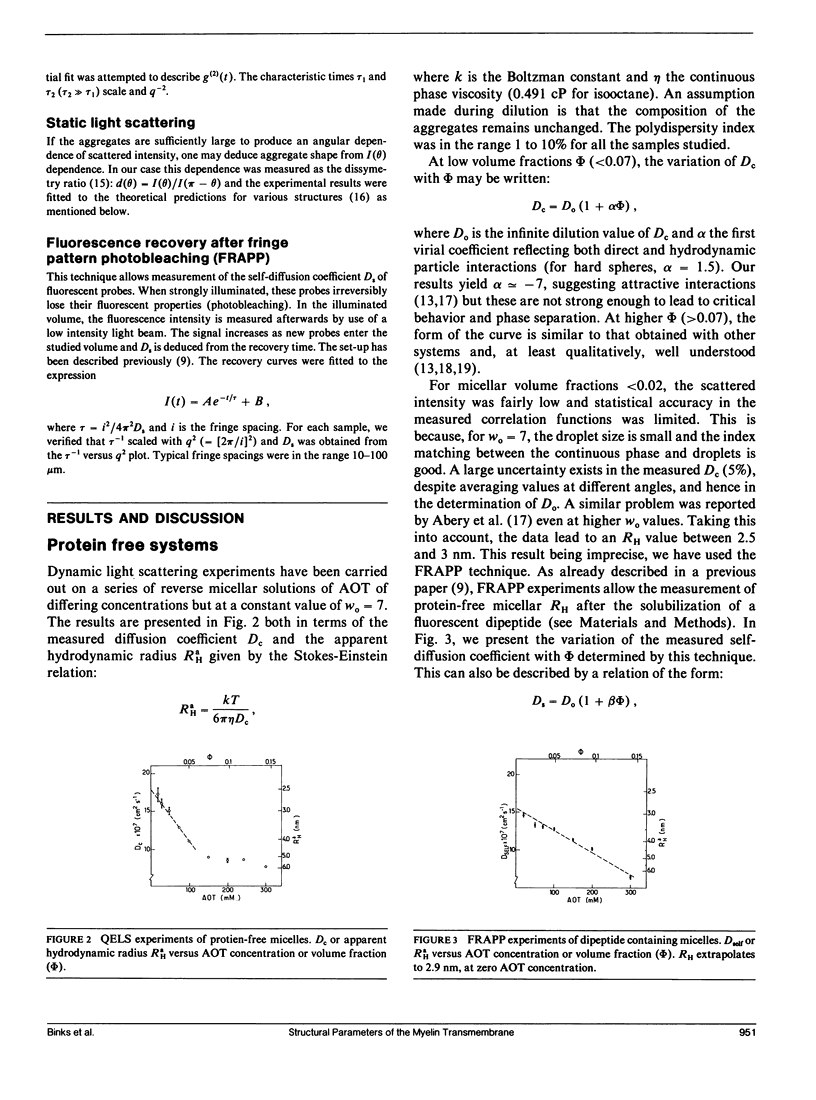
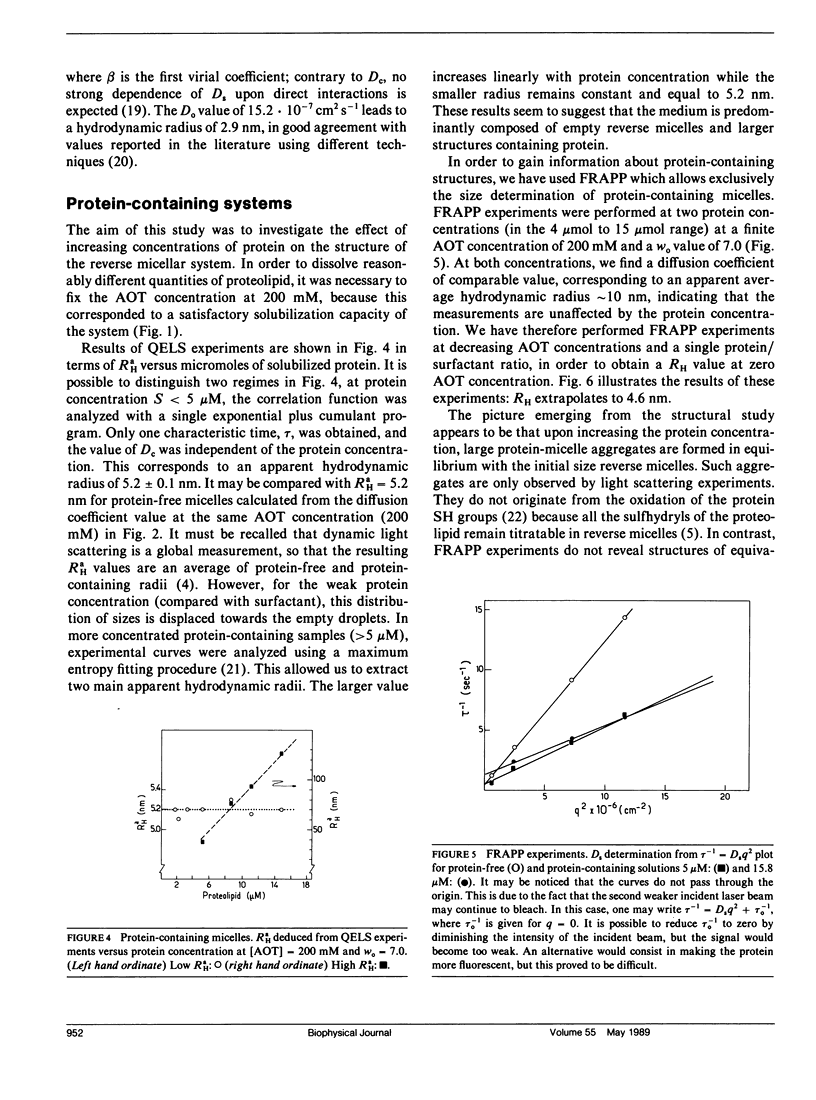
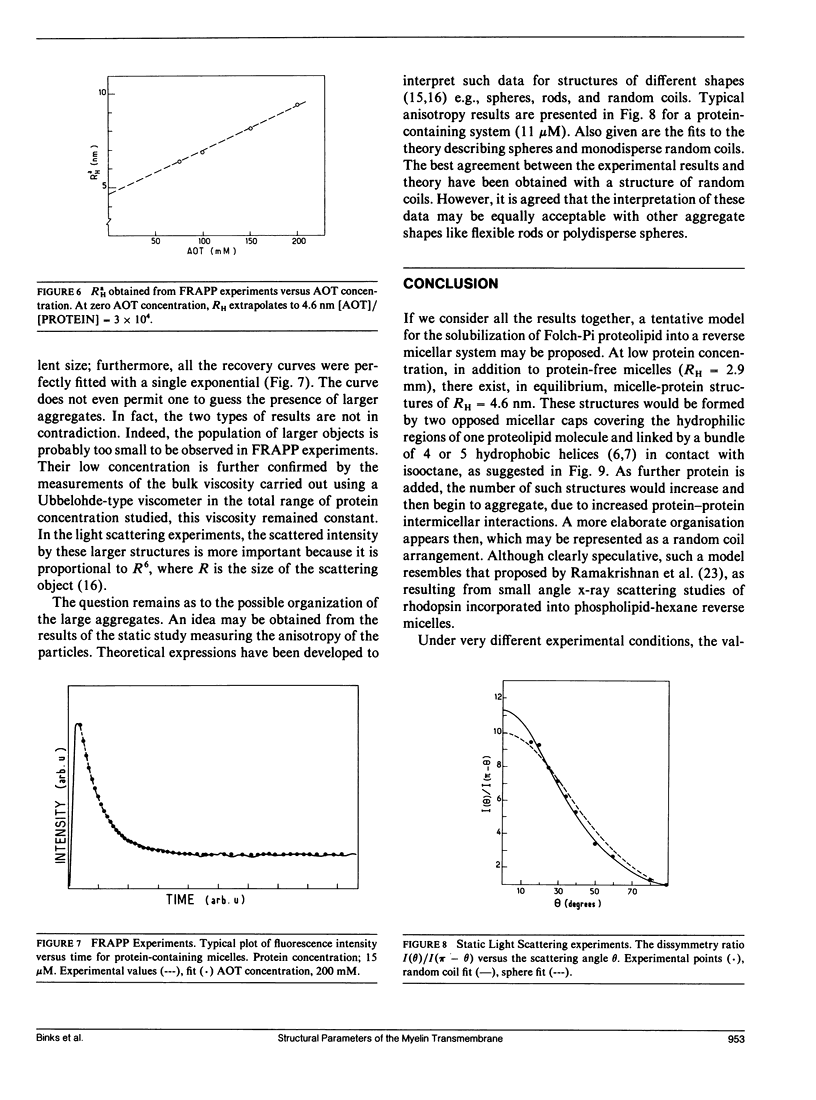
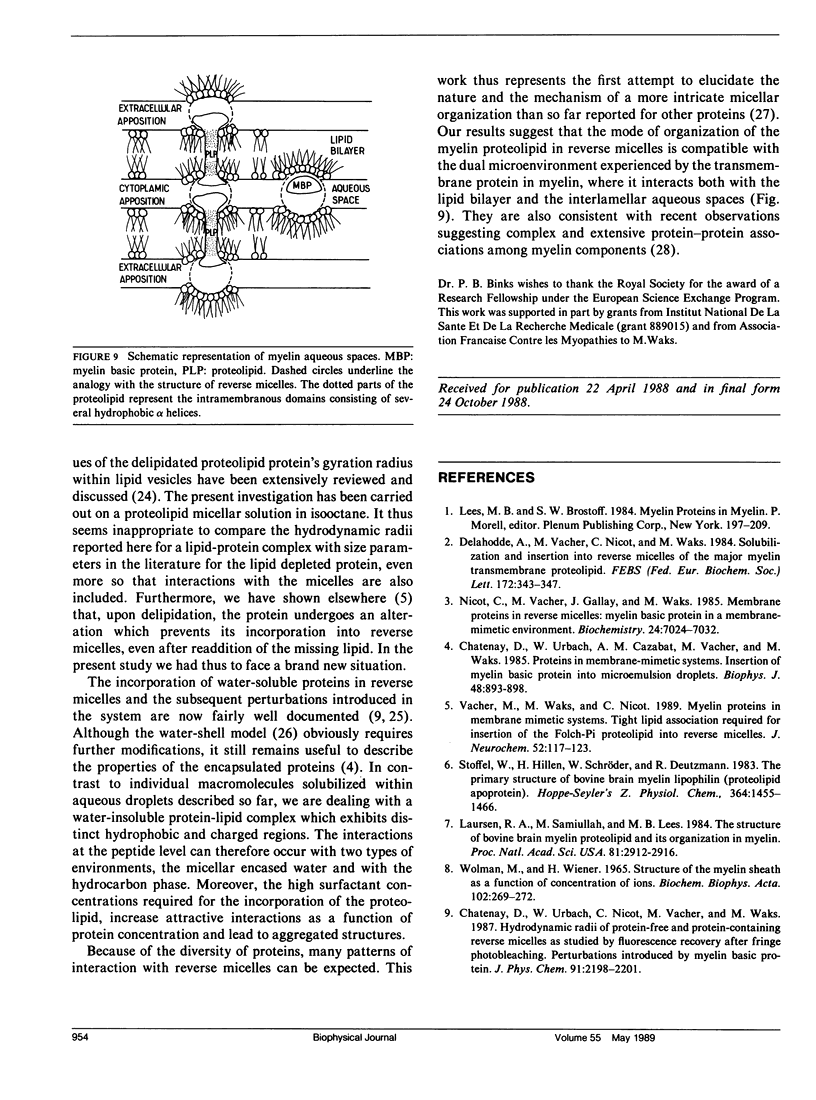
Abstract
The Folch-Pi proteolipid is the most abundant structural protein from the central nervous system myelin. This protein-lipid complex, normally insoluble in water, requires only a small amount of water for solubilization in reverse micelles of sodium bis (2-ethylhexyl) sulfosuccinate (AOT) in isooctane. The characterization of the proteolipid-free and proteolipid-containing micelles was undertaken by light scattering and fluorescence recovery after fringe pattern photobleaching (FRAPP) experiments. Quasi elastic light scattering (QELS) was carried out at a high (200 mM) AOT concentration, at low water-to-surfactant mole ratio (Wo = 7) and at increasing protein occupancy. Two apparent hydrodynamic radii, differing tenfold in size, were obtained from correlation functions. The smaller one (RaH = 5.2 nm) remains constant and corresponds to that measured for protein-free micelles. The larger one increases linearly with protein concentration. In contrast, FRAPP measurements of self-diffusion coefficients were found unaffected by the proteolipid concentration. Accordingly, they have been performed at constant protein/surfactant mole ratios. The equivalent RH, extrapolated to zero AOT concentration for protein-free reverse micelles (2.9 nm) and in the presence of the proteolipid (4.6 nm), do not reveal the mode of organization previously suggested by QELS measurements. The complex picture emerging from this work represents a first step in the characterization of an integral membrane protein in reverse micelles.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chatenay D., Urbach W., Cazabat A. M., Vacher M., Waks M. Proteins in membrane mimetic systems. Insertion of myelin basic protein into microemulsion droplets. Biophys J. 1985 Dec;48(6):893–898. doi: 10.1016/S0006-3495(85)83851-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatenay D, Urbach W, Cazabat AM, Langevin D. Onset of droplet aggregation from self-diffusion measurements in microemulsions. Phys Rev Lett. 1985 May 20;54(20):2253–2256. doi: 10.1103/PhysRevLett.54.2253. [DOI] [PubMed] [Google Scholar]
- Garel J. R. pK changes of ionizable reporter groups as an index of conformational changes in proteins. A study of fluorescein-labelled ribonuclease A. Eur J Biochem. 1976 Nov 1;70(1):179–189. doi: 10.1111/j.1432-1033.1976.tb10968.x. [DOI] [PubMed] [Google Scholar]
- Laursen R. A., Samiullah M., Lees M. B. The structure of bovine brain myelin proteolipid and its organization in myelin. Proc Natl Acad Sci U S A. 1984 May;81(9):2912–2916. doi: 10.1073/pnas.81.9.2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luisi P. L., Magid L. J. Solubilization of enzymes and nucleic acids in hydrocarbon micellar solutions. CRC Crit Rev Biochem. 1986;20(4):409–474. doi: 10.3109/10409238609081999. [DOI] [PubMed] [Google Scholar]
- Nicot C., Vacher M., Vincent M., Gallay J., Waks M. Membrane proteins in reverse micelles: myelin basic protein in a membrane-mimetic environment. Biochemistry. 1985 Nov 19;24(24):7024–7032. doi: 10.1021/bi00345a041. [DOI] [PubMed] [Google Scholar]
- Pereyra P. M., Horvath E., Braun P. E. Triton X-100 extractions of central nervous system myelin indicate a possible role for the minor myelin proteins in the stability in lamellae. Neurochem Res. 1988 Jun;13(6):583–595. doi: 10.1007/BF00973301. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan V. R., Darszon A., Montal M. A small angle x-ray scattering study of a rhodopsin-lipid complex in hexane. J Biol Chem. 1983 Apr 25;258(8):4857–4860. [PubMed] [Google Scholar]
- Stoffel W., Hillen H., Schröder W., Deutzmann R. The primary structure of bovine brain myelin lipophilin (proteolipid apoprotein). Hoppe Seylers Z Physiol Chem. 1983 Oct;364(10):1455–1466. doi: 10.1515/bchm2.1983.364.2.1455. [DOI] [PubMed] [Google Scholar]
- Vacher M., Waks M., Nicot C. Myelin proteins in reverse micelles: tight lipid association required for insertion of the Folch-Pi proteolipid into a membrane-mimetic system. J Neurochem. 1989 Jan;52(1):117–123. doi: 10.1111/j.1471-4159.1989.tb10905.x. [DOI] [PubMed] [Google Scholar]
- Vacher M., Waks M., Nicot C. The thiol groups of the Folch-Pi protein from bovine white matter. Exposure, reactivity and significance. Biochem J. 1984 Feb 15;218(1):197–202. doi: 10.1042/bj2180197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolman M., Wiener H. Structure of the myelin sheath as a function of concentration of ions. Biochim Biophys Acta. 1965 May 25;102(1):269–279. doi: 10.1016/0926-6585(65)90219-0. [DOI] [PubMed] [Google Scholar]


