Abstract
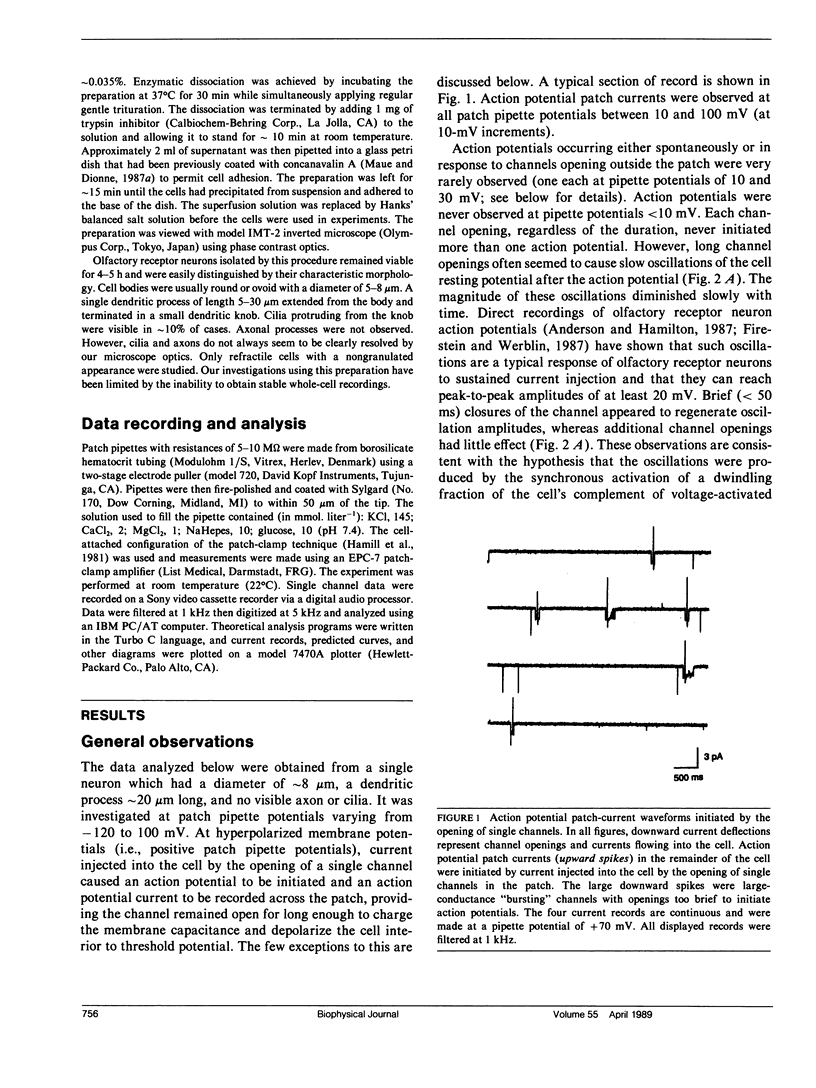
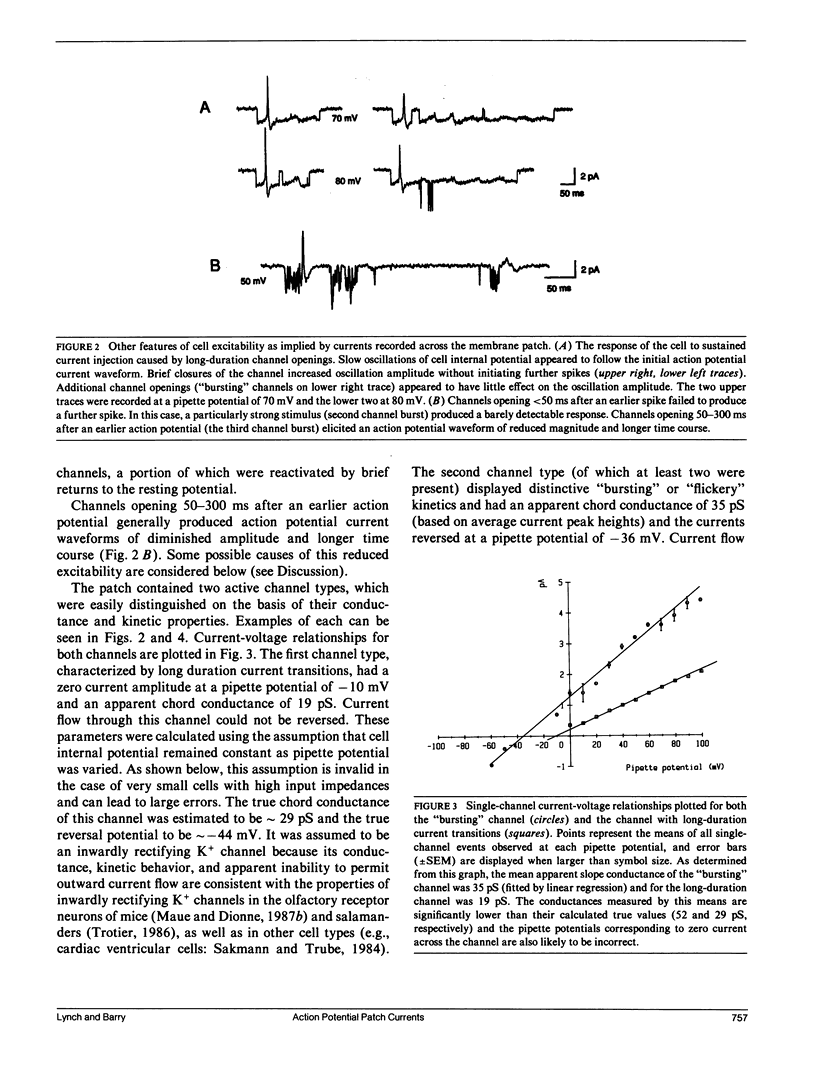
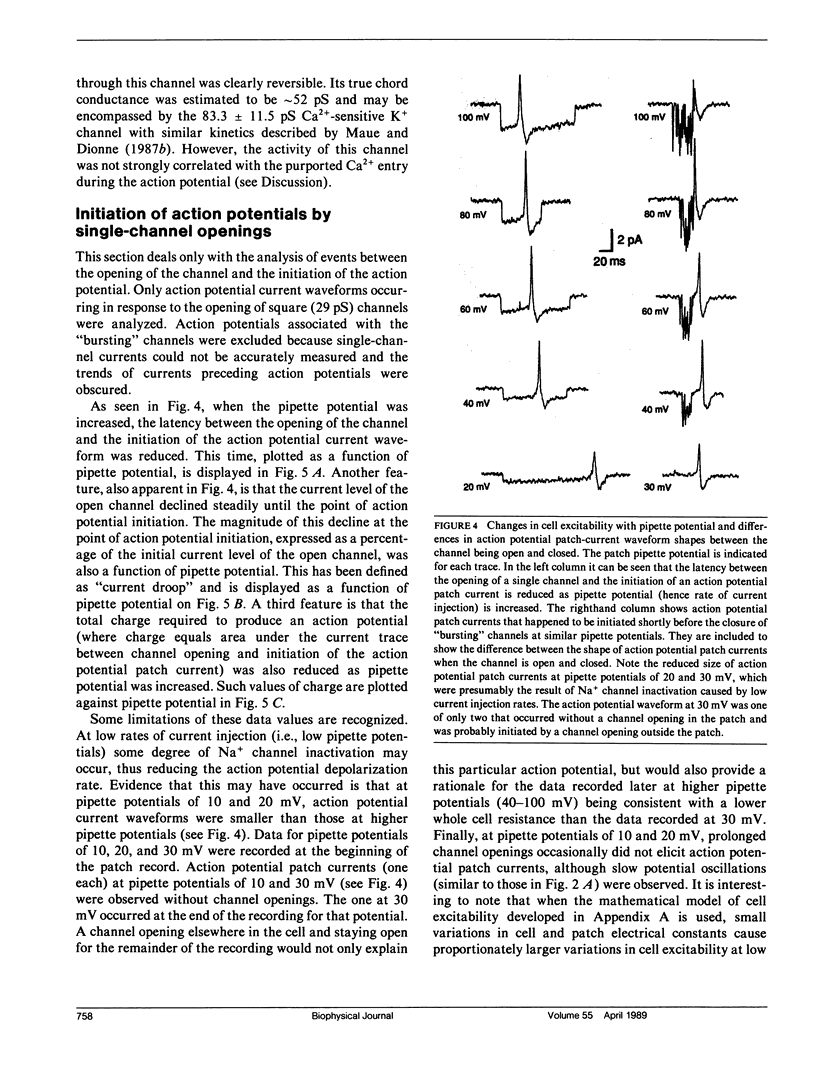
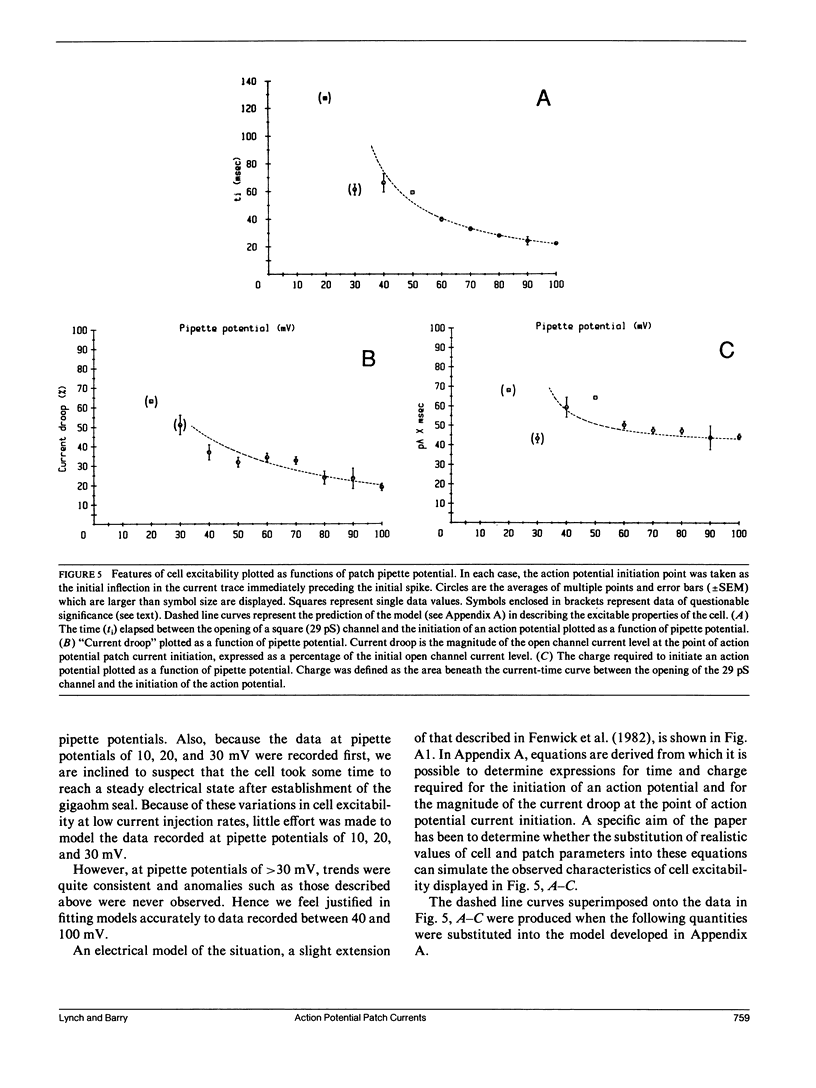
Rat olfactory receptor neurons were enzymatically dissociated and studied with the cell-attached configuration of the patch-clamp technique. Biphasic current waveforms induced across the membrane patch by intracellular action potentials were observed in approximately 5% of cells studied. In one cell in particular, current injected by the opening of a single channel initiated an action potential in the remainder of the cell each time the channel opened. A conventional type of electrical model of the cell and patch allowed the accurate modeling of cell excitability. The same model was used to explain the shape of the action potential current waveforms induced across the patch. The analysis indicated that the whole cell resistance (Ro) was approximately 40 G omega and the membrane capacitance (Co) was close to the standard value of 1 microF.cm-2. In addition, the threshold potential change necessary to initiate an action potential (Vth) was approximately 13 mV and a minimum current injection of 1 pA was required to depolarize the cell to spike threshold. When the smaller size of mammalian receptors are taken into account, membrane electrical properties were found to be consistent with those of salamander cells investigated by others using whole-cell recording. The analysis also revealed possible errors in the determination of single-channel conductances and reversal potentials by cell-attached recording from small cells.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P. A., Hamilton K. A. Intracellular recordings from isolated salamander olfactory receptor neurons. Neuroscience. 1987 Apr;21(1):167–173. doi: 10.1016/0306-4522(87)90330-7. [DOI] [PubMed] [Google Scholar]
- Ashcroft F. M., Harrison D. E., Ashcroft S. J. Glucose induces closure of single potassium channels in isolated rat pancreatic beta-cells. 1984 Nov 29-Dec 5Nature. 312(5993):446–448. doi: 10.1038/312446a0. [DOI] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. A patch-clamp study of bovine chromaffin cells and of their sensitivity to acetylcholine. J Physiol. 1982 Oct;331:577–597. doi: 10.1113/jphysiol.1982.sp014393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestein S., Werblin F. S. Gated currents in isolated olfactory receptor neurons of the larval tiger salamander. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6292–6296. doi: 10.1073/pnas.84.17.6292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischmeister R., Ayer R. K., Jr, DeHaan R. L. Some limitations of the cell-attached patch clamp technique: a two-electrode analysis. Pflugers Arch. 1986 Jan;406(1):73–82. doi: 10.1007/BF00582957. [DOI] [PubMed] [Google Scholar]
- Frings S., Lindemann B. Odorant response of isolated olfactory receptor cells is blocked by amiloride. J Membr Biol. 1988 Nov;105(3):233–243. doi: 10.1007/BF01871000. [DOI] [PubMed] [Google Scholar]
- Gesteland R. C. Speculations on receptor cells as analyzers and filters. Experientia. 1986 Mar 15;42(3):287–291. doi: 10.1007/BF01942509. [DOI] [PubMed] [Google Scholar]
- Getchell T. V. Functional properties of vertebrate olfactory receptor neurons. Physiol Rev. 1986 Jul;66(3):772–818. doi: 10.1152/physrev.1986.66.3.772. [DOI] [PubMed] [Google Scholar]
- Getchell T. V., Shepherd G. M. Adaptive properties of olfactory receptors analysed with odour pulses of varying durations. J Physiol. 1978 Sep;282:541–560. doi: 10.1113/jphysiol.1978.sp012480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hedlund B., Masukawa L. M., Shepherd G. M. Excitable properties of olfactory receptor neurons. J Neurosci. 1987 Aug;7(8):2338–2343. [PMC free article] [PubMed] [Google Scholar]
- Labarca P., Simon S. A., Anholt R. R. Activation by odorants of a multistate cation channel from olfactory cilia. Proc Natl Acad Sci U S A. 1988 Feb;85(3):944–947. doi: 10.1073/pnas.85.3.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancet D. Vertebrate olfactory reception. Annu Rev Neurosci. 1986;9:329–355. doi: 10.1146/annurev.ne.09.030186.001553. [DOI] [PubMed] [Google Scholar]
- Maue R. A., Dionne V. E. Patch-clamp studies of isolated mouse olfactory receptor neurons. J Gen Physiol. 1987 Jul;90(1):95–125. doi: 10.1085/jgp.90.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maue R. A., Dionne V. E. Preparation of isolated mouse olfactory receptor neurons. Pflugers Arch. 1987 Jul;409(3):244–250. doi: 10.1007/BF00583472. [DOI] [PubMed] [Google Scholar]
- Sakmann B., Trube G. Conductance properties of single inwardly rectifying potassium channels in ventricular cells from guinea-pig heart. J Physiol. 1984 Feb;347:641–657. doi: 10.1113/jphysiol.1984.sp015088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotier D. A patch-clamp analysis of membrane currents in salamander olfactory receptor cells. Pflugers Arch. 1986 Dec;407(6):589–595. doi: 10.1007/BF00582636. [DOI] [PubMed] [Google Scholar]


