Abstract
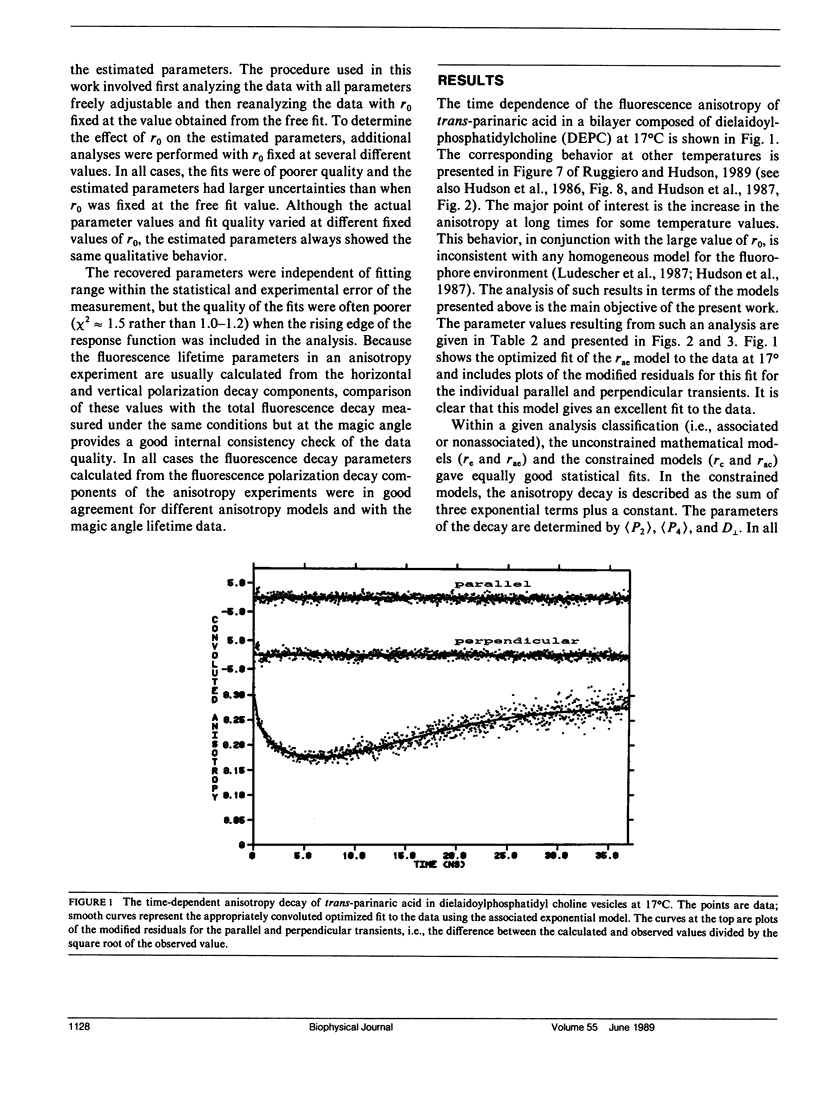
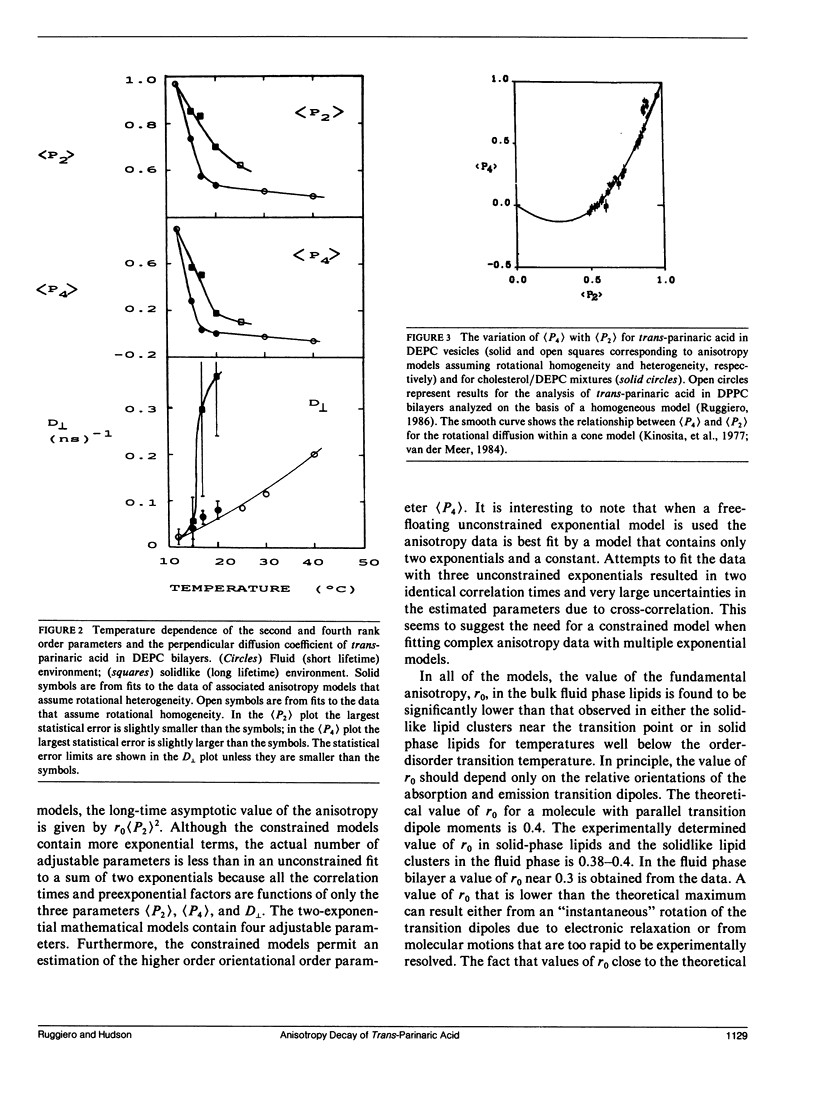
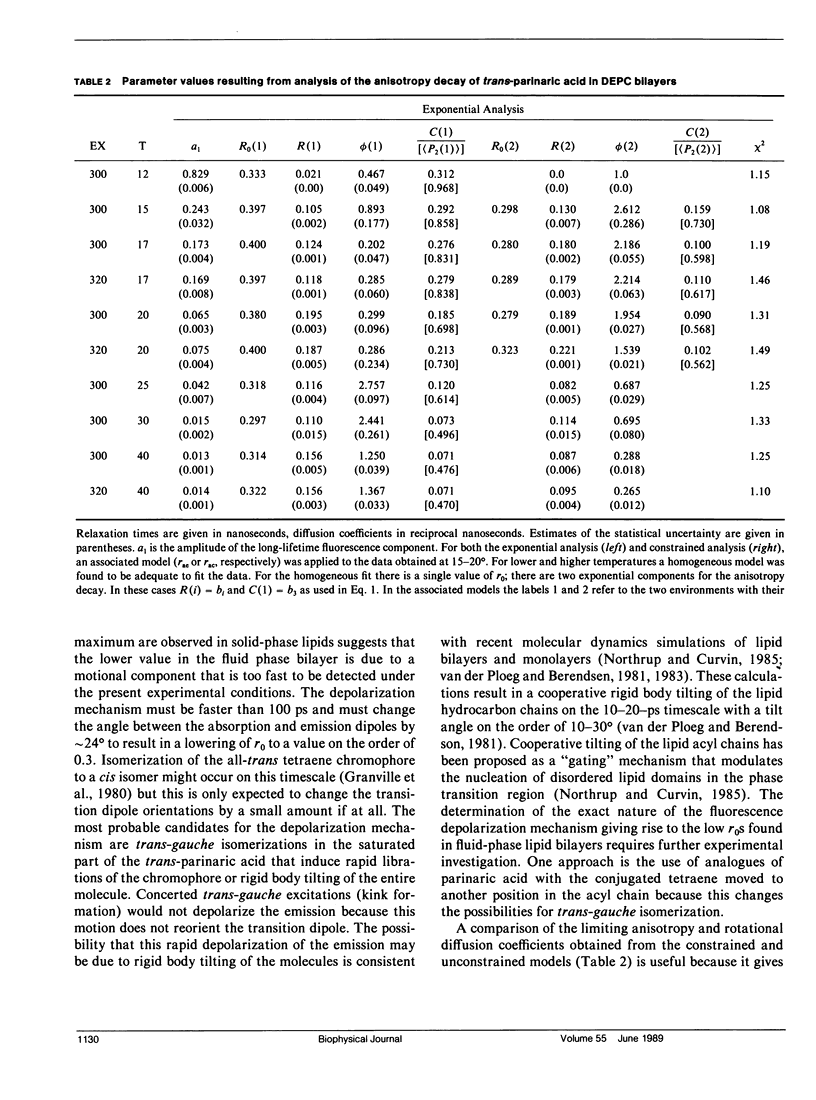
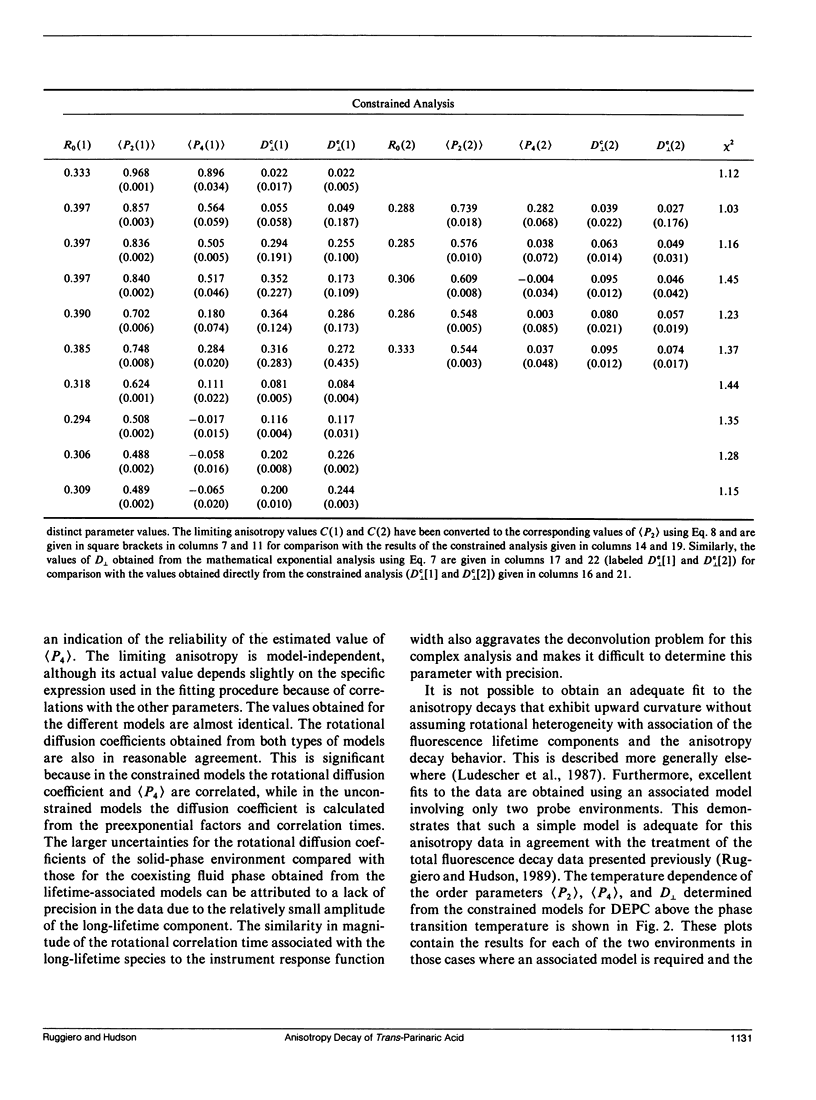
An analysis is presented of the complex anisotropy behavior of trans-parinaric acid in single component DEPC lipid bilayers. It is shown that a model involving two species with distinct lifetime and motional behavior is required, and is adequate, to explain the observed data. In particular, the observed increase in the anisotropy at long times demonstrates the presence of a species with a long fluorescence lifetime that has a high anisotropy. The time dependence of the anisotropy for these two environments is treated using both a purely mathematical sum of exponentials and a constrained fit based on an approximate solution of the anisotropic diffusion problem. In this latter model the anisotropy is described in terms of the second and fourth rank order parameters, (P2) and (P4), and a single dynamical parameter, D1, the perpendicular diffusion coefficient for this uniaxial probe. The parameters of both models are accurately determined from the fits to the data when two environments coexist and an association is made between lifetime components and distinct rotational sites. The values of the parameters obtained demonstrate the "solid-like" and "fluidlike" nature of these two coexisting environments.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ameloot M., Hendrickx H., Herreman W., Pottel H., Van Cauwelaert F., van der Meer W. Effect of orientational order on the decay of the fluorescence anisotropy in membrane suspensions. Experimental verification on unilamellar vesicles and lipid/alpha-lactalbumin complexes. Biophys J. 1984 Oct;46(4):525–539. doi: 10.1016/S0006-3495(84)84050-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claesens F., Rigler R. Conformational dynamics of the anticodon loop in yeast tRNAPhe as sensed by the fluorescence of wybutine. Eur Biophys J. 1986;13(6):331–342. doi: 10.1007/BF00265669. [DOI] [PubMed] [Google Scholar]
- Cross A. J., Fleming G. R. Analysis of time-resolved fluorescence anisotropy decays. Biophys J. 1984 Jul;46(1):45–56. doi: 10.1016/S0006-3495(84)83997-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale R. E., Chen L. A., Brand L. Rotational relaxation of the "microviscosity" probe diphenylhexatriene in paraffin oil and egg lecithin vesicles. J Biol Chem. 1977 Nov 10;252(21):7500–7510. [PubMed] [Google Scholar]
- Granville M. F., Holtom G. R., Kohler B. E. cis-trans photoisomerization of 1,3,5,7-octatetraene in n-hexane at 4.2 K. Proc Natl Acad Sci U S A. 1980 Jan;77(1):31–33. doi: 10.1073/pnas.77.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinvald A., Steinberg I. Z. On the analysis of fluorescence decay kinetics by the method of least-squares. Anal Biochem. 1974 Jun;59(2):583–598. doi: 10.1016/0003-2697(74)90312-1. [DOI] [PubMed] [Google Scholar]
- Heyn M. P. Determination of lipid order parameters and rotational correlation times from fluorescence depolarization experiments. FEBS Lett. 1979 Dec 15;108(2):359–364. doi: 10.1016/0014-5793(79)80564-5. [DOI] [PubMed] [Google Scholar]
- Isenberg I., Dyson R. D. The analysis of fluorescence decay by a method of moments. Biophys J. 1969 Nov;9(11):1337–1350. doi: 10.1016/S0006-3495(69)86456-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jähnig F. Critical effects from lipid-protein interaction in membranes. I. Theoretical description. Biophys J. 1981 Nov;36(2):329–345. doi: 10.1016/S0006-3495(81)84735-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jähnig F. Critical effects from lipid-protein interaction in membranes. II. Interpretation of experimental results. Biophys J. 1981 Nov;36(2):347–357. doi: 10.1016/S0006-3495(81)84736-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jähnig F. Structural order of lipids and proteins in membranes: evaluation of fluorescence anisotropy data. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6361–6365. doi: 10.1073/pnas.76.12.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawato S., Kinosita K., Jr, Ikegami A. Dynamic structure of lipid bilayers studied by nanosecond fluorescence techniques. Biochemistry. 1977 May 31;16(11):2319–2324. doi: 10.1021/bi00630a002. [DOI] [PubMed] [Google Scholar]
- Kawato S., Kinosita K., Jr, Ikegami A. Effect of cholesterol on the molecular motion in the hydrocarbon region of lecithin bilayers studied by nanosecond fluorescence techniques. Biochemistry. 1978 Nov 14;17(23):5026–5031. doi: 10.1021/bi00616a026. [DOI] [PubMed] [Google Scholar]
- Kinosita K., Jr, Ikegami A., Kawato S. On the wobbling-in-cone analysis of fluorescence anisotropy decay. Biophys J. 1982 Feb;37(2):461–464. doi: 10.1016/S0006-3495(82)84692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinosita K., Jr, Kawato S., Ikegami A. A theory of fluorescence polarization decay in membranes. Biophys J. 1977 Dec;20(3):289–305. doi: 10.1016/S0006-3495(77)85550-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer J. M., Esker M. W., Pathmamanoharan C., Wiersema P. H. Vesicles of variable diameter prepared by a modified injection method. Biochemistry. 1977 Aug 23;16(17):3932–3935. doi: 10.1021/bi00636a033. [DOI] [PubMed] [Google Scholar]
- Ludescher R. D., Peting L., Hudson S., Hudson B. Time-resolved fluorescence anisotropy for systems with lifetime and dynamic heterogeneity. Biophys Chem. 1987 Oct;28(1):59–75. doi: 10.1016/0301-4622(87)80075-3. [DOI] [PubMed] [Google Scholar]
- Mitaku S., Date T. Anomalies of nanosecond ultrasonic relaxation in the lipid bilayer transition. Biochim Biophys Acta. 1982 Jun 14;688(2):411–421. doi: 10.1016/0005-2736(82)90352-2. [DOI] [PubMed] [Google Scholar]
- Mitaku S., Ikegami A., Sakanishi A. Ultrasonic studies of lipid bilayer. Phase transition in synthetic phosphatidylcholine liposomes. Biophys Chem. 1978 Sep;8(4):295–304. doi: 10.1016/0301-4622(78)80012-x. [DOI] [PubMed] [Google Scholar]
- Mitaku S., Jippo T., Kataoka R. Thermodynamic properties of the lipid bilayer transition. Pseudocritical phenomena. Biophys J. 1983 May;42(2):137–144. doi: 10.1016/S0006-3495(83)84379-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitaku S., Okano K. Ultrasonic measurements of two-component lipid bilayer suspensions. Biophys Chem. 1981 Oct;14(2):147–158. doi: 10.1016/0301-4622(81)85015-6. [DOI] [PubMed] [Google Scholar]
- Mouritsen O. G., Zuckermann M. J. Softening of lipid bilayers. Eur Biophys J. 1985;12(2):75–86. doi: 10.1007/BF00260430. [DOI] [PubMed] [Google Scholar]
- Nagle J. F. Theory of lipid monolayer and bilayer phase transitions: effect of headgroup interactions. J Membr Biol. 1976;27(3):233–250. doi: 10.1007/BF01869138. [DOI] [PubMed] [Google Scholar]
- Rigler R., Ehrenberg M. Molecular interactions and structure as analysed by fluorescence relaxation spectroscopy. Q Rev Biophys. 1973 May;6(2):139–199. doi: 10.1017/s003358350000113x. [DOI] [PubMed] [Google Scholar]
- Ruggiero A., Hudson B. Critical density fluctuations in lipid bilayers detected by fluorescence lifetime heterogeneity. Biophys J. 1989 Jun;55(6):1111–1124. doi: 10.1016/S0006-3495(89)82908-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklar L. A., Hudson B. S. Conjugated polyene fatty acids as fluorescent membrane probes: model system studies. J Supramol Struct. 1976;4(4):449–465. doi: 10.1002/jss.400040404. [DOI] [PubMed] [Google Scholar]
- Sklar L. A., Hudson B. S., Simoni R. D. Conjugated polyene fatty acids as fluorescent probes: synthetic phospholipid membrane studies. Biochemistry. 1977 Mar 8;16(5):819–828. doi: 10.1021/bi00624a002. [DOI] [PubMed] [Google Scholar]
- Sklar L. A., Hudson B. S., Simoni R. D. Conjugated polyene fatty acids as membrane probes: preliminary characterization. Proc Natl Acad Sci U S A. 1975 May;72(5):1649–1653. doi: 10.1073/pnas.72.5.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolber P. K., Hudson B. S. Bilayer acyl chain dynamics and lipid-protein interaction: the effect of the M13 bacteriophage coat protein on the decay of the fluorescence anisotropy of parinaric acid. Biophys J. 1982 Jan;37(1):253–262. doi: 10.1016/S0006-3495(82)84674-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolber P. K., Hudson B. S. Fluorescence lifetime and time-resolved polarization anisotropy studies of acyl chain order and dynamics in lipid bilayers. Biochemistry. 1981 May 12;20(10):2800–2810. doi: 10.1021/bi00513a015. [DOI] [PubMed] [Google Scholar]
- van der Meer W., Pottel H., Herreman W., Ameloot M., Hendrickx H., Schröder H. Effect of orientational order on the decay of the fluorescence anisotropy in membrane suspensions. A new approximate solution of the rotational diffusion equation. Biophys J. 1984 Oct;46(4):515–523. doi: 10.1016/S0006-3495(84)84049-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


