Abstract
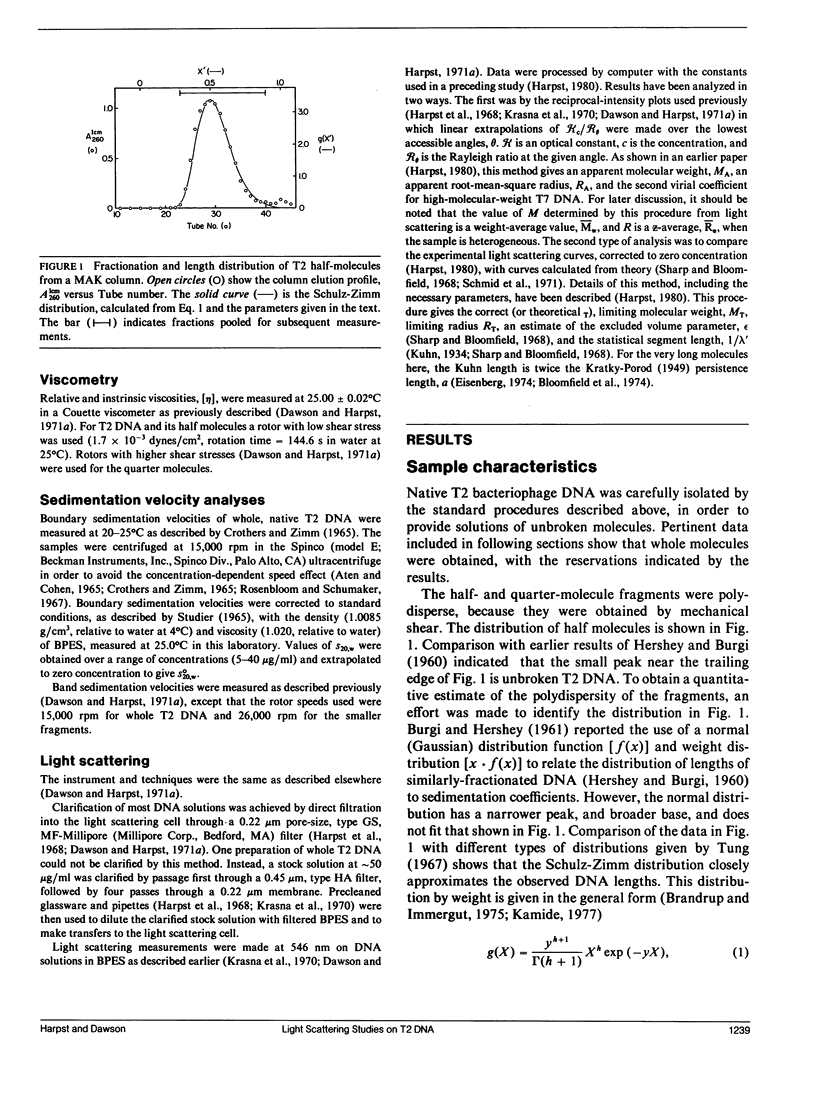
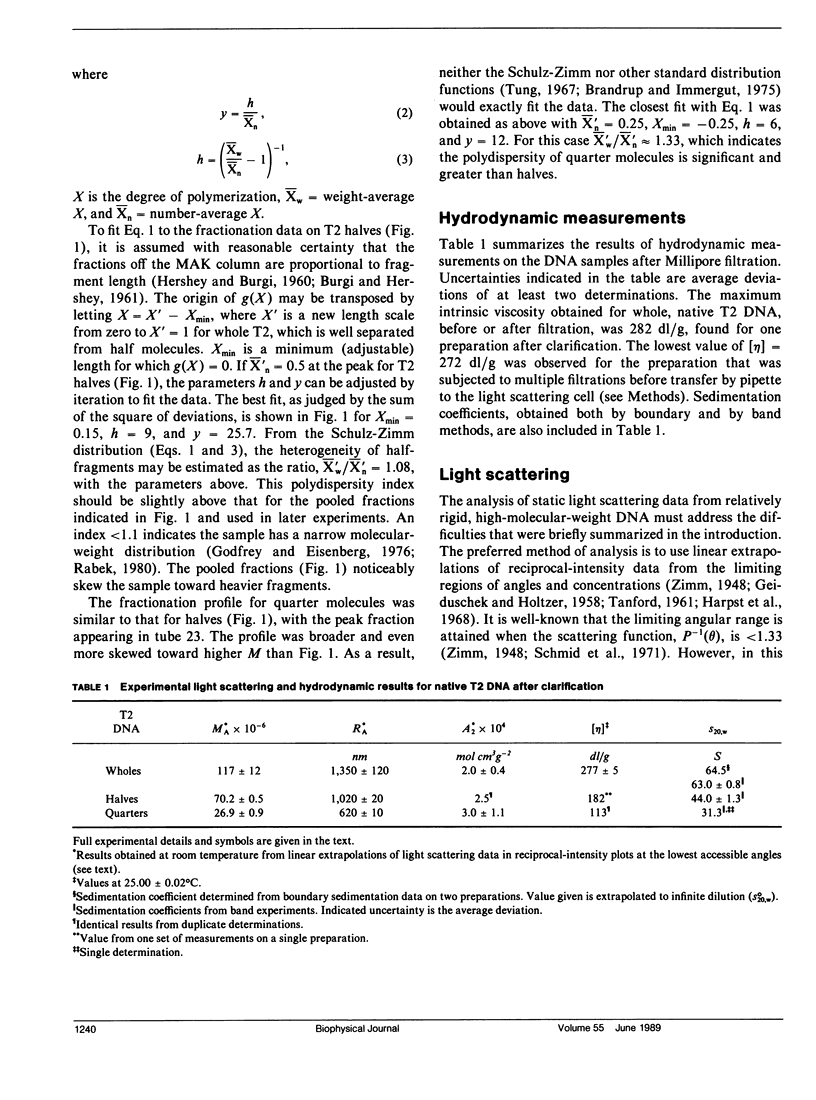
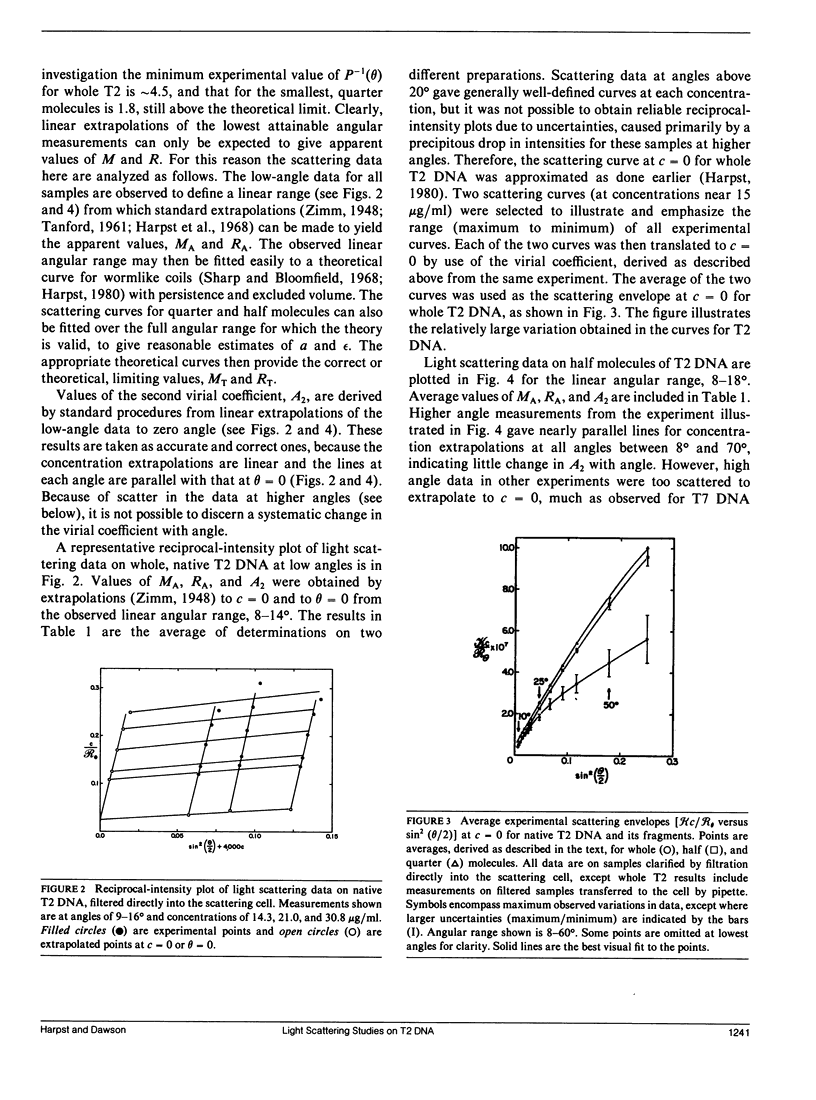
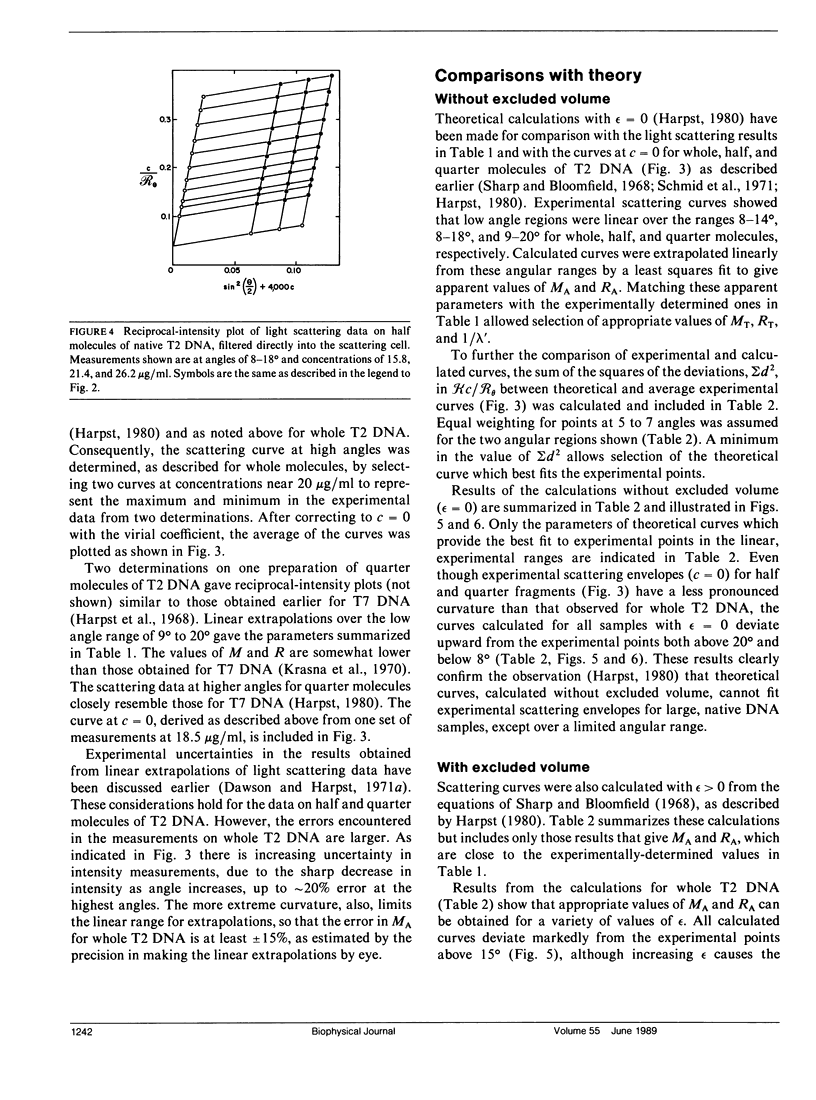
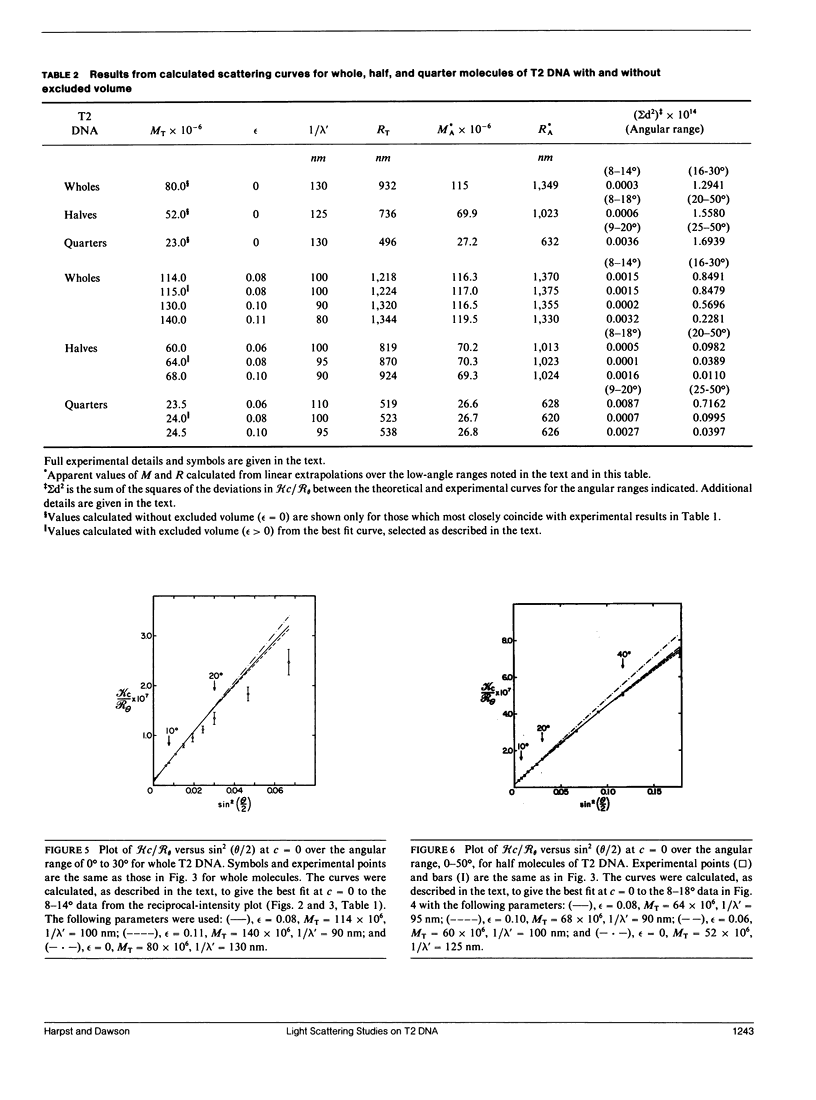
Static light scattering measurements have been made at angles as low as 8 degrees on whole, half, and quarter molecules of native, T2 bacteriophage DNA in 0.195 M Na+. The fragments were obtained by high-speed stirring of the native DNA, and fractionated on methylated-albumin-kieselguhr columns. Accompanying measurements of sedimentation coefficients and intrinsic viscosities were made. Because linear extrapolations of light scattering data above 8 degrees for these samples were suspect, the measurements were analyzed by fitting curves calculated from the theory of wormlike coils to experimental curves at c = 0. Results showed that the excluded volume parameter, epsilon, must be used in analyzing the scattering curves; a reasonable value of epsilon was 0.08, in agreement with that found for T7 DNA (Harpst, J. A. 1980. Biophys. Chem. 11:295-302). The persistence length of all three DNAs in this paper was 50 +/- 5 nm, showed no dependence on molecular weight, but was somewhat below that reported previously for T7 DNA (60 nm). Theoretical curves calculated with the preceding parameters had a clear upward curvature in scattering envelopes below 8 degrees for quarter and half molecules, but such curvature was minimal for whole T2 DNA, so that linear extrapolations of experimental data above 8 degrees gave a molecular weight and root-mean-square radius which were nearly the same as those from theory. The molecular weight and radius for whole T2, derived from the comparison of theory and experiment, were 115 X 10(6) and 1,224 nm, respectively. The measurements on T2 DNA were clearly at the upper limit of current techniques.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aten J. B., Cohen J. A. Sedimentation-viscosity studies of high molecular weight DNA. J Mol Biol. 1965 Jul;12(3):537–548. doi: 10.1016/s0022-2836(65)80311-4. [DOI] [PubMed] [Google Scholar]
- BURGI E., HERSHEY A. D. A relative molecular weight series derived from the nucleic acid of bacteriophage T2. J Mol Biol. 1961 Aug;3:458–472. doi: 10.1016/s0022-2836(61)80058-2. [DOI] [PubMed] [Google Scholar]
- Borochov N., Eisenberg H. Conformation of LiDNA in solutions of LiCl. Biopolymers. 1984 Sep;23(9):1757–1769. doi: 10.1002/bip.360230910. [DOI] [PubMed] [Google Scholar]
- Bowen B. C., Zimm B. H. Molecular weight of T2 NaDNA from viscoelasticity. Biophys Chem. 1978 Jan;7(4):235–252. doi: 10.1016/0301-4622(78)85001-7. [DOI] [PubMed] [Google Scholar]
- Cairney K. L., Harrington R. E. Flow birefringence of T7 phage DNA: dependence on salt concentration. Biopolymers. 1982 May;21(5):923–934. doi: 10.1002/bip.360210506. [DOI] [PubMed] [Google Scholar]
- Crothers D. M., Zimm B. H. Viscosity and sedimentation of the DNA from bacteriophages T2 and T7 and the relation to molecular weight. J Mol Biol. 1965 Jul;12(3):525–536. doi: 10.1016/s0022-2836(65)80310-2. [DOI] [PubMed] [Google Scholar]
- Dawson J. R., Harpst J. A. Light scattering and hydrodynamic properties of linear and circular bacteriophage lambda DNA. Biopolymers. 1971;10(12):2499–2508. doi: 10.1002/bip.360101211. [DOI] [PubMed] [Google Scholar]
- Eigner J., Doty P. The native, denatured and renatured states of deoxyribonucleic acid. J Mol Biol. 1965 Jul;12(3):549–580. doi: 10.1016/s0022-2836(65)80312-6. [DOI] [PubMed] [Google Scholar]
- FRASER D., JERREL E. A. The amino acid composition of T3 bacteriophage. J Biol Chem. 1953 Nov;205(1):291–295. [PubMed] [Google Scholar]
- Freifelder D. Molecular weights of coliphages and coliphage DNA. IV. Molecular weights of DNA from bacteriophages T4, T5 and T7 and the general problem of determination of M. J Mol Biol. 1970 Dec 28;54(3):567–577. doi: 10.1016/0022-2836(70)90127-0. [DOI] [PubMed] [Google Scholar]
- GEIDUSCHEK E. P., HOLTZER A. Application of light scattering to biological systems: deoxyribonucleic acid and the muscle proteins. Adv Biol Med Phys. 1958;6:431–551. doi: 10.1016/b978-1-4832-3112-9.50014-1. [DOI] [PubMed] [Google Scholar]
- Godfrey J. E., Eisenberg H. The flexibility of low molecular weight double-stranded DNA as a function of length. I. Light scattering measurements and the estimation of persistence lengths from light scattering, sedimentation and viscosity. Biophys Chem. 1976 Sep;5(3):301–318. doi: 10.1016/0301-4622(76)80042-7. [DOI] [PubMed] [Google Scholar]
- Godfrey J. E. The flexibility of low molecular weight double-stranded DNA as a function of length. I. Isolation andphysical characterization of seven fractions. Biophys Chem. 1976 Sep;5(3):285–299. doi: 10.1016/0301-4622(76)80041-5. [DOI] [PubMed] [Google Scholar]
- Hagerman P. J. Investigation of the flexibility of DNA using transient electric birefringence. Biopolymers. 1981 Jul;20(7):1503–1535. doi: 10.1002/bip.1981.360200710. [DOI] [PubMed] [Google Scholar]
- Harpst J. A. Analysis of low angle light scattering results from T7 DNA. Biophys Chem. 1980 Apr;11(2):295–302. doi: 10.1016/0301-4622(80)80032-9. [DOI] [PubMed] [Google Scholar]
- Harpst J. A., Krasna A. I., Zimm B. H. Molecular weight of T7 and calf thymus DNA by low-angle light scattering. Biopolymers. 1968 Apr;6(4):595–603. doi: 10.1002/bip.1968.360060413. [DOI] [PubMed] [Google Scholar]
- KRASNA A. I., HARPST J. A. CLARIFICATION OF NATIVE DNA SOLUTIONS BY FILTRATION. Proc Natl Acad Sci U S A. 1964 Jan;51:36–40. doi: 10.1073/pnas.51.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam Z., Borochov N., Eisenberg H. Dependence of laser light scattering of DNA on NaCl concentration. Biopolymers. 1981 Dec;20(12):2671–2690. doi: 10.1002/bip.1981.360201213. [DOI] [PubMed] [Google Scholar]
- Krasna A. I., Dawson J. R., Harpst J. A. Characterization of acid-denatured DNA by low-angle light scattering. Biopolymers. 1970;9(9):1017–1028. doi: 10.1002/bip.1970.360090905. [DOI] [PubMed] [Google Scholar]
- Lang D. Molecular weights of coliphages and coliphage DNA. 3. Contour length and molecular weight of DNA from bacteriophages T4, T5 and T7, and from bovine papilloma virus. J Mol Biol. 1970 Dec 28;54(3):557–565. doi: 10.1016/0022-2836(70)90126-9. [DOI] [PubMed] [Google Scholar]
- MANDELL J. D., HERSHEY A. D. A fractionating column for analysis of nucleic acids. Anal Biochem. 1960 Jun;1:66–77. doi: 10.1016/0003-2697(60)90020-8. [DOI] [PubMed] [Google Scholar]
- Manning G. S. The molecular theory of polyelectrolyte solutions with applications to the electrostatic properties of polynucleotides. Q Rev Biophys. 1978 May;11(2):179–246. doi: 10.1017/s0033583500002031. [DOI] [PubMed] [Google Scholar]
- Maret G., Weill G. Magnetic birefringence study of the electrostatic and intrinsic persistence length of DNA. Biopolymers. 1983 Dec;22(12):2727–2744. doi: 10.1002/bip.360221215. [DOI] [PubMed] [Google Scholar]
- RUBENSTEIN I., THOMAS C. A., Jr, HERSHEY A. D. The molecular weights of T2 bacteriophage DNA and its first and second breakage products. Proc Natl Acad Sci U S A. 1961 Aug;47:1113–1122. doi: 10.1073/pnas.47.8.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinert K. E., Strassburger J., Triebel H. Molecular weights and hydrodynamic properties for homogeneous native DNA, derived from diffusion, sedimentation, and viscosity measurements on polydisperse samples. Biopolymers. 1971;10(2):285–307. doi: 10.1002/bip.360100206. [DOI] [PubMed] [Google Scholar]
- Rizzo V., Schellman J. Flow dichroism of T7 DNA as a function of salt concentration. Biopolymers. 1981 Oct;20(10):2143–2163. doi: 10.1002/bip.1981.360201009. [DOI] [PubMed] [Google Scholar]
- Roberts T. M., Lauer G. D., Klotz L. C. Physical studies on DNA from "primitive" eucaryotes. CRC Crit Rev Biochem. 1975;3(4):349–449. [PubMed] [Google Scholar]
- Rosenbloom J., Schumaker V. Molecular weight dependence of the rotor speed induced aggregation of deoxyribonucleic acid. Biochemistry. 1967 Jan;6(1):276–283. doi: 10.1021/bi00853a043. [DOI] [PubMed] [Google Scholar]
- Ross P. D., Scruggs R. L. Viscosity study of DNA. II. The effect of simple salt concentration on the viscosity of high molecular weight DNA and application of viscometry to the study of DNA isolated from T4 and T5 bacteriophage mutants. Biopolymers. 1968;6(8):1005–1018. doi: 10.1002/bip.1968.360060802. [DOI] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Schmid C. W., Rinehart F. P., Hearst J. E. Statistical length of DNA from light scattering. Biopolymers. 1971;10(5):883–893. doi: 10.1002/bip.360100511. [DOI] [PubMed] [Google Scholar]
- Sharp P., Bloomfield V. A. Light scattering from wormlike chains with excluded volume effects. Biopolymers. 1968;6(8):1201–1211. doi: 10.1002/bip.1968.360060814. [DOI] [PubMed] [Google Scholar]
- Voordouw G., Kam Z., Borochov N., Eisenberg H. Isolation and physical studies of the intact supercoiled, the open circular and the linear forms of ColE1-plasmid DNA. Biophys Chem. 1978 May;8(2):171–189. doi: 10.1016/0301-4622(78)80008-8. [DOI] [PubMed] [Google Scholar]
- Weissman M., Schindler H., Feher G. Determination of molecular weights by fluctuation spectroscopy: application to DNA. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2776–2780. doi: 10.1073/pnas.73.8.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]


