Abstract
1. Receptive field centres of 144 sustained and transient retinal ganglion cells were mapped in cats under light pentobarbitone anaesthesia.
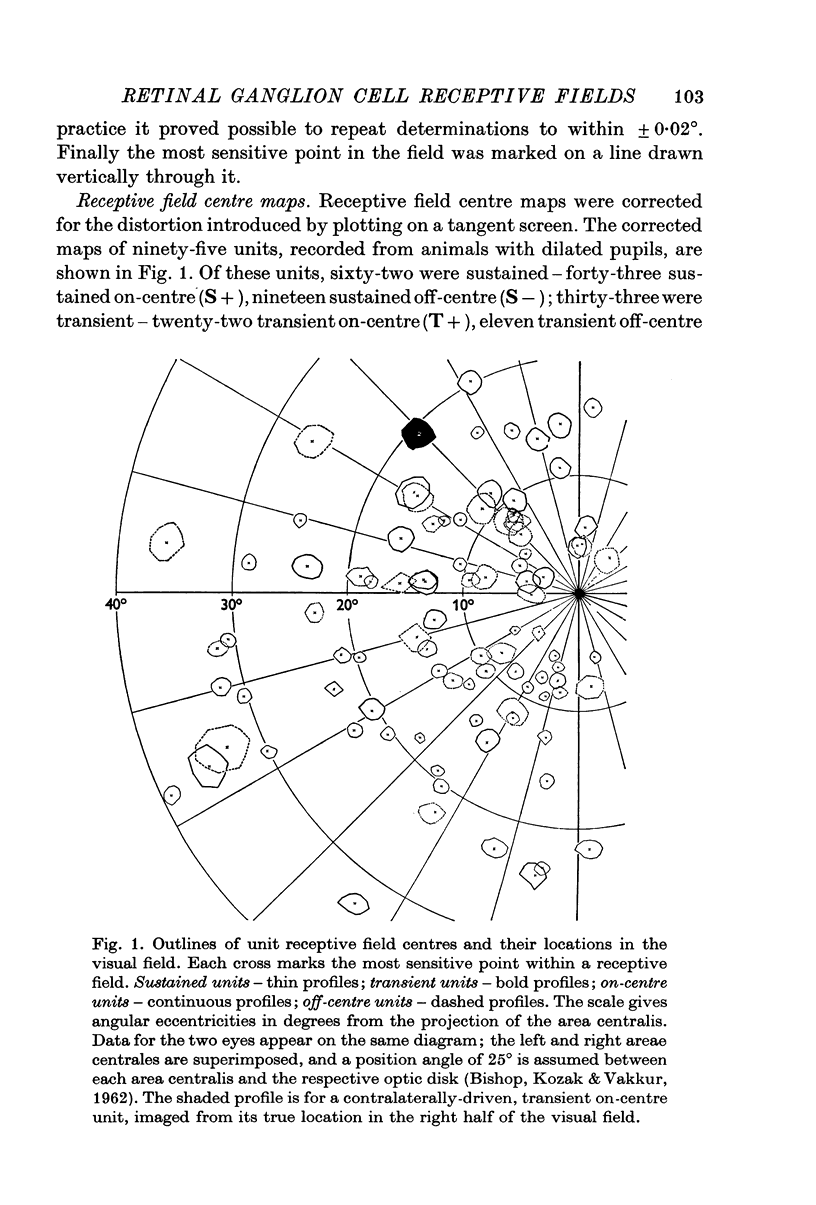
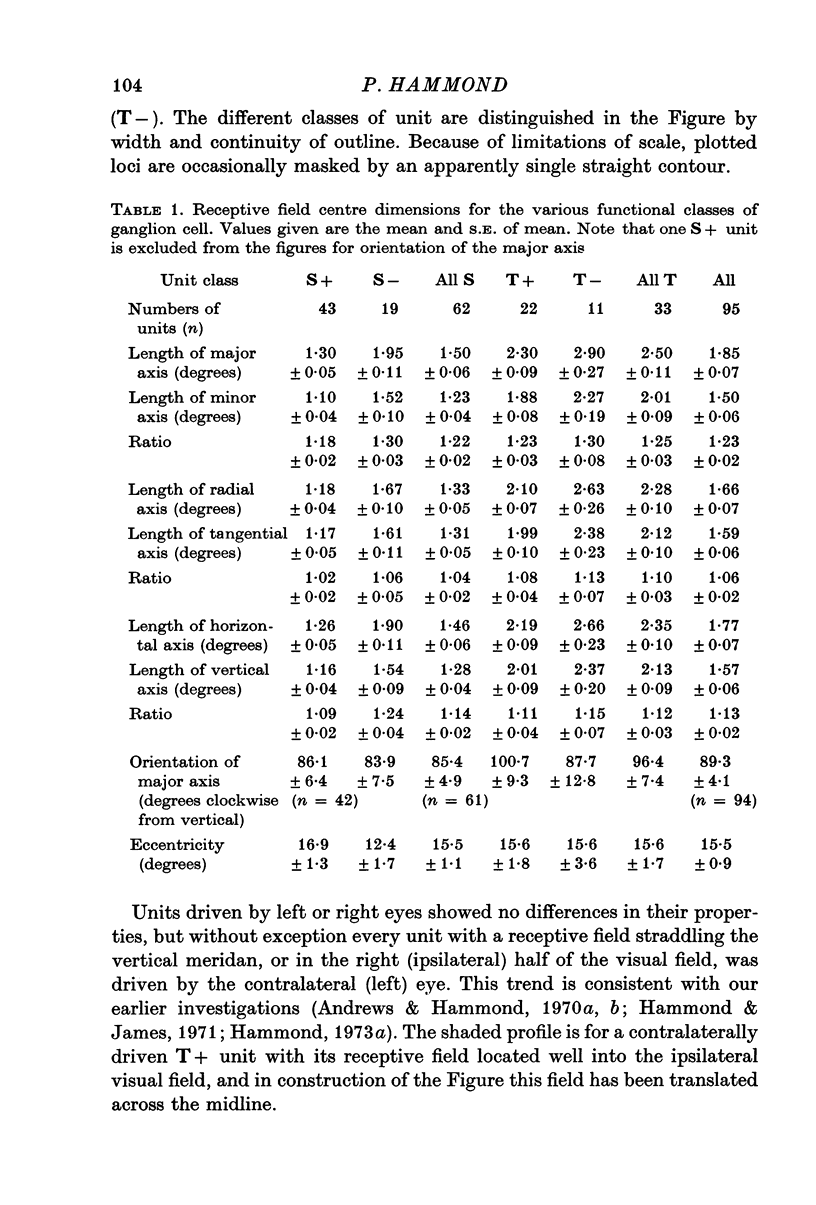
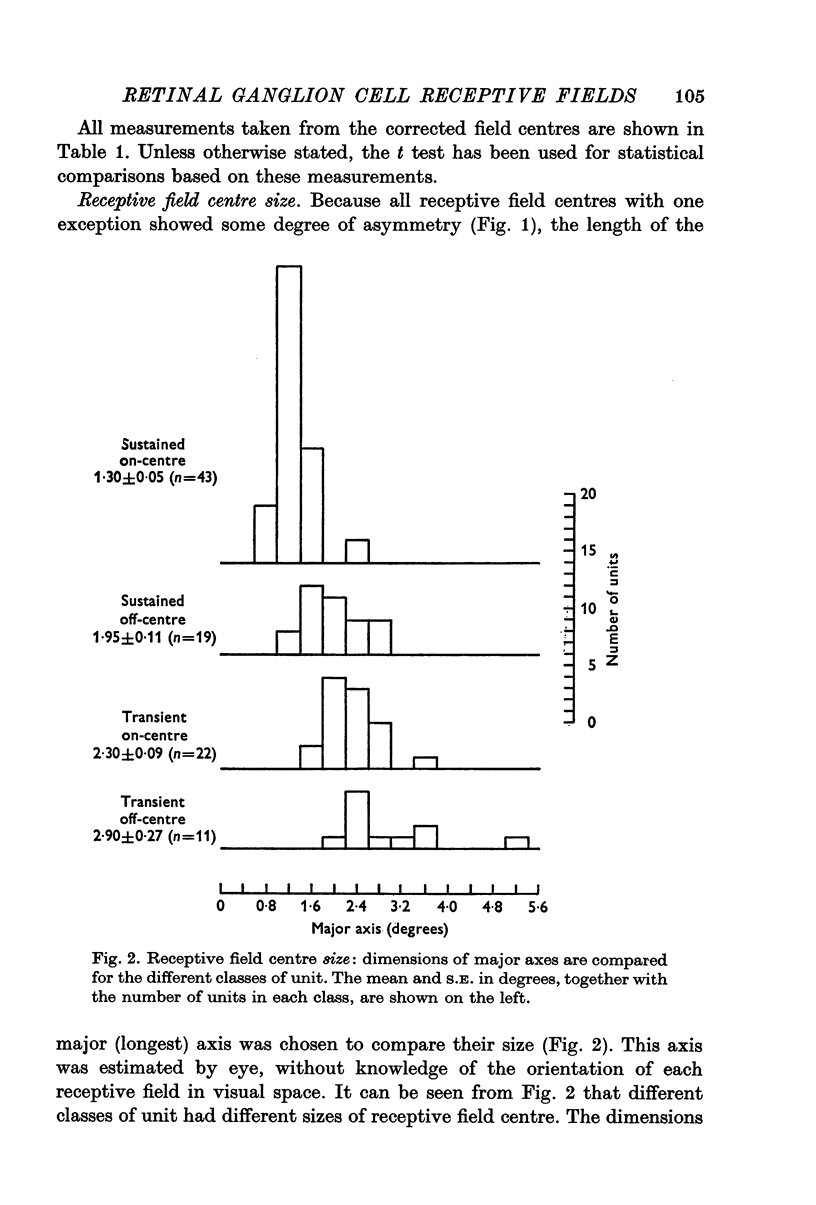
2. Sustained on-centre, sustained off-centre, transient on-centre and transient off-centre cells had different mean sizes of receptive field centre, with some overlap between their distributions.
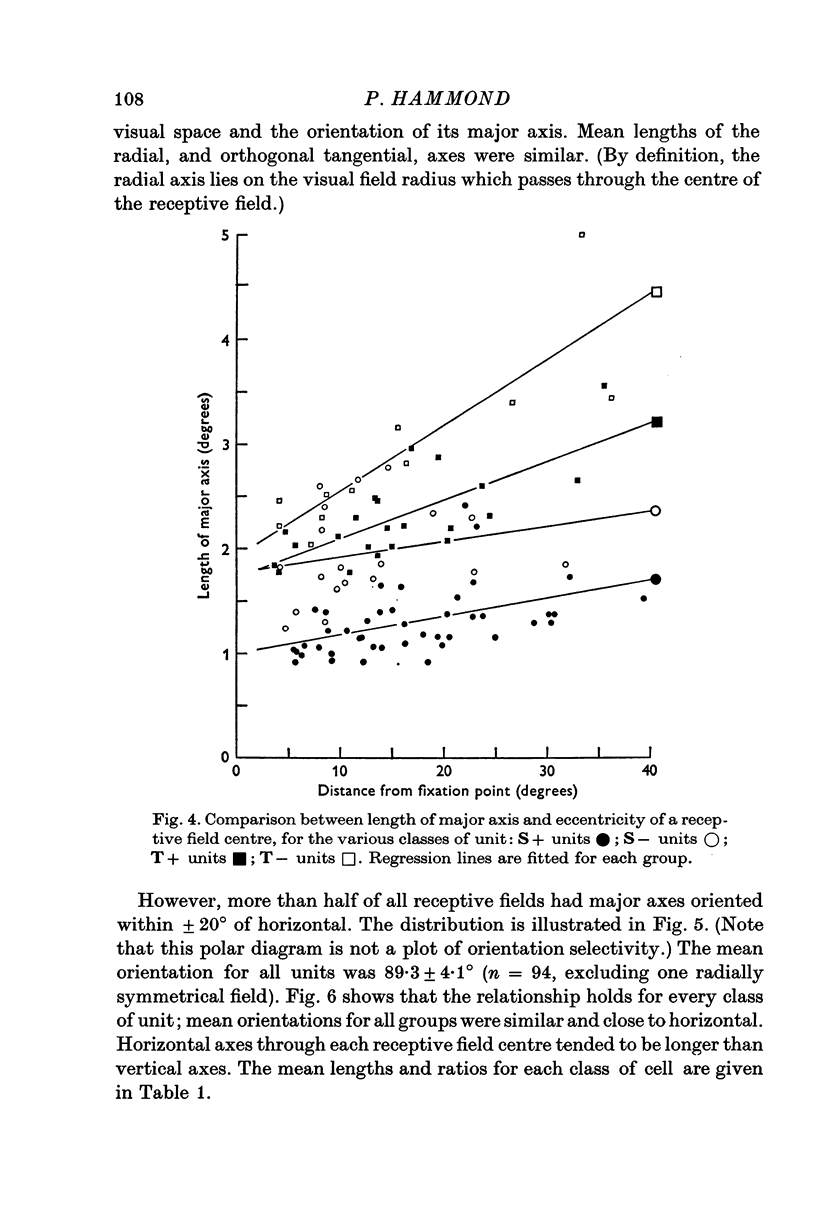
3. For each class of cell, central fields had the smallest field-centres; progressively larger field-centres were encountered more peripherally.
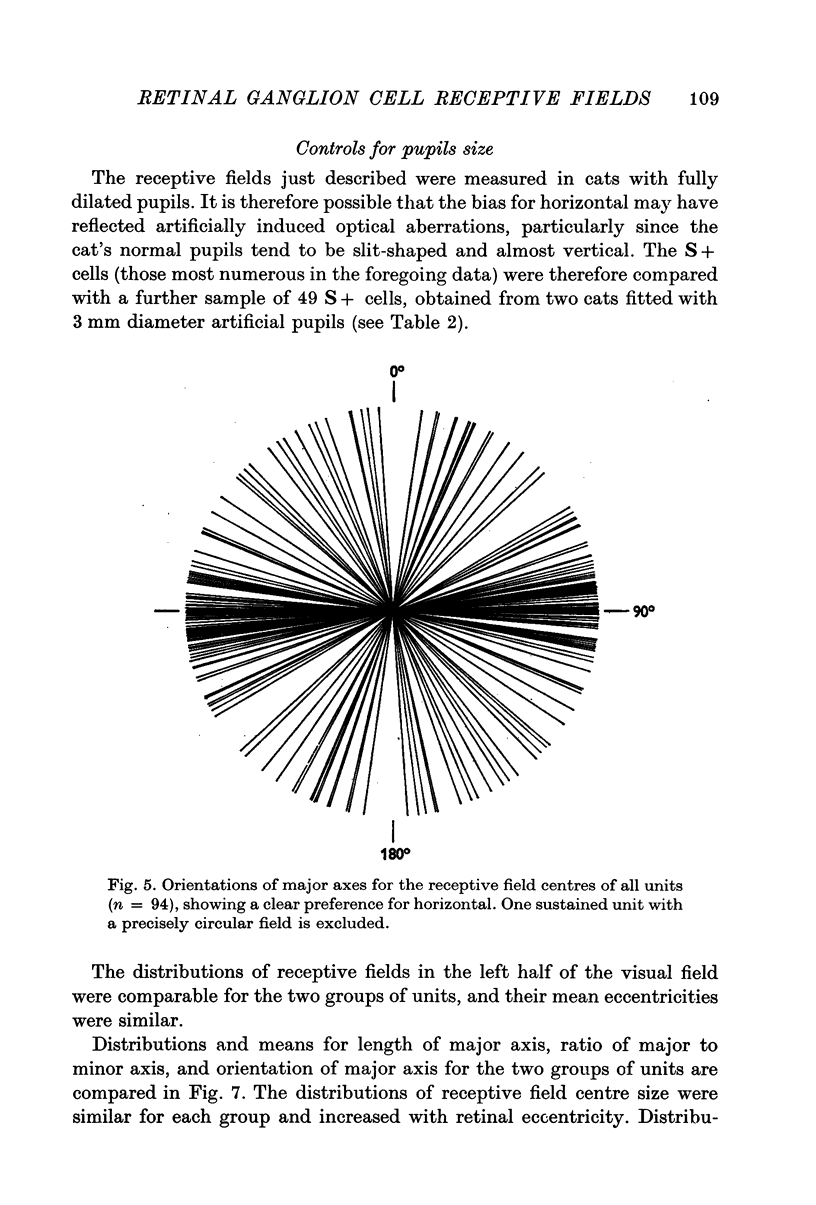
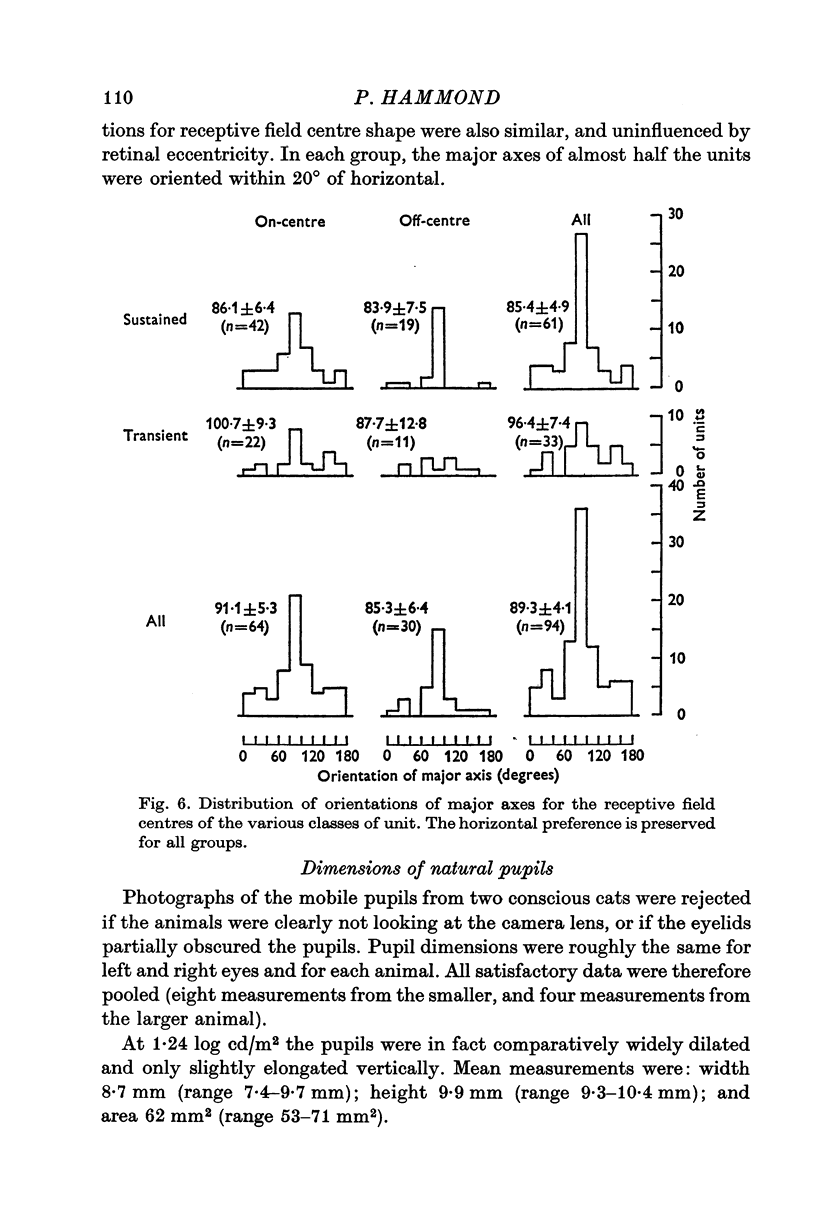
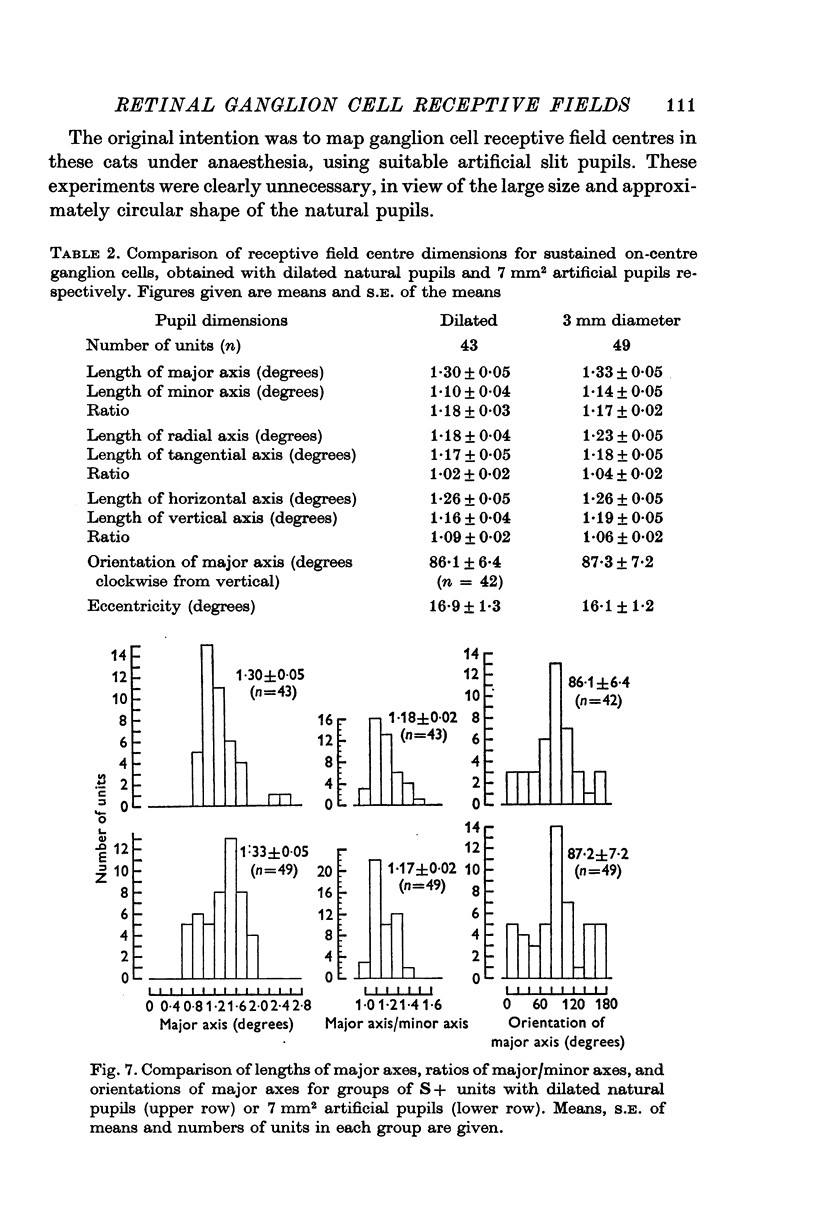
4. All classes of ganglion cells tended to have slightly elliptical receptive field centres. Major axes of over half of all receptive fields were oriented within 20° of horizontal. These trends were independent of pupil dimensions, or of receptive field eccentricity or position in the visual field. The results almost certainly reflect asymmetry in retinal wiring.
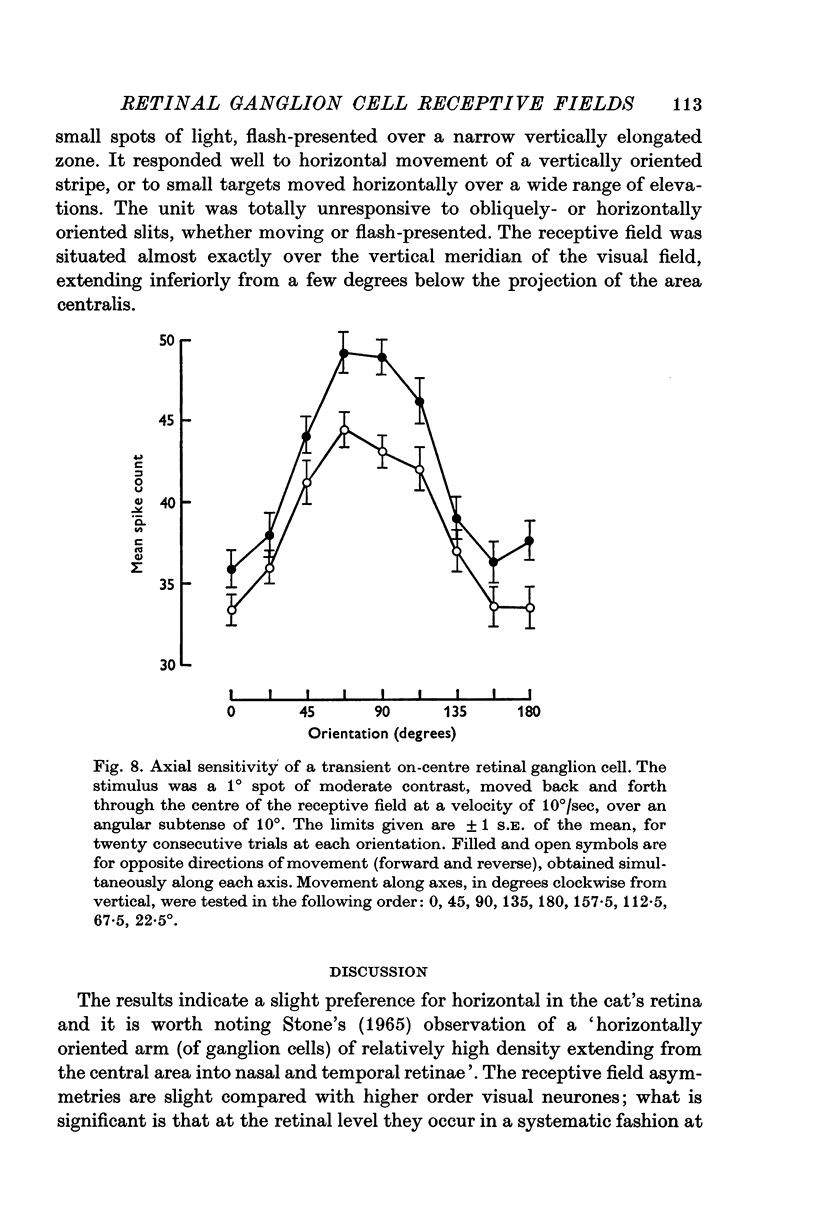
5. Two cells of thirty-nine tested were sensitive to axis of motion; in both cases the preferred and major axis were horizontal. A further cell was orientation specific.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews D. P., Hammond P. Mesopic increment threshold spectral sensitivity of single optic tract fibres in the cat: cone-rod interaction. J Physiol. 1970 Jul;209(1):65–81. doi: 10.1113/jphysiol.1970.sp009156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews D. P., Hammond P. Suprathreshold spectral properties of single optic tract fibres in cat, under mesopic adaptation: cone-rod interaction. J Physiol. 1970 Jul;209(1):83–103. doi: 10.1113/jphysiol.1970.sp009157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARLOW H. B., FITZHUGH R., KUFFLER S. W. Change of organization in the receptive fields of the cat's retina during dark adaptation. J Physiol. 1957 Aug 6;137(3):338–354. doi: 10.1113/jphysiol.1957.sp005817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BISHOP P. O., KOZAK W., VAKKUR G. J. Some quantitative aspects of the cat's eye: axis and plane of reference, visual field co-ordinates and optics. J Physiol. 1962 Oct;163:466–502. doi: 10.1113/jphysiol.1962.sp006990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow H. B., Levick W. R. Changes in the maintained discharge with adaptation level in the cat retina. J Physiol. 1969 Jun;202(3):699–718. doi: 10.1113/jphysiol.1969.sp008836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow H. B., Levick W. R., Westheimer G. Computer-plotted receptive fields. Science. 1966 Nov 18;154(3751):920–920. doi: 10.1126/science.154.3751.920-a. [DOI] [PubMed] [Google Scholar]
- Berman N., Cynader M. Comparison of receptive-field organization of the superior colliculus in Siamese and normal cats. J Physiol. 1972 Jul;224(2):363–389. doi: 10.1113/jphysiol.1972.sp009900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonds A. B., Enroth-Cugell C., Pinto L. H. Image quality of the cat eye measured during retinal ganglion cell experiments. J Physiol. 1972 Jan;220(2):383–401. doi: 10.1113/jphysiol.1972.sp009713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boycott B. B., Wässle H. The morphological types of ganglion cells of the domestic cat's retina. J Physiol. 1974 Jul;240(2):397–419. doi: 10.1113/jphysiol.1974.sp010616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. E., Major D. Cat retinal ganglion cell dendritic fields. Exp Neurol. 1966 May;15(1):70–78. doi: 10.1016/0014-4886(66)90035-5. [DOI] [PubMed] [Google Scholar]
- Campbell F. W., Cleland B. G., Cooper G. F., Enroth-Cugell C. The angular selectivity of visual cortical cells to moving gratings. J Physiol. 1968 Sep;198(1):237–250. doi: 10.1113/jphysiol.1968.sp008604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell F. W., Green D. G. Optical and retinal factors affecting visual resolution. J Physiol. 1965 Dec;181(3):576–593. doi: 10.1113/jphysiol.1965.sp007784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Dubin M. W., Levick W. R. Sustained and transient neurones in the cat's retina and lateral geniculate nucleus. J Physiol. 1971 Sep;217(2):473–496. doi: 10.1113/jphysiol.1971.sp009581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Levick W. R. Physiology of cat retinal ganglion cells. Invest Ophthalmol. 1972 May;11(5):285–291. [PubMed] [Google Scholar]
- Cleland B. G., Levick W. R., Sanderson K. J. Properties of sustained and transient ganglion cells in the cat retina. J Physiol. 1973 Feb;228(3):649–680. doi: 10.1113/jphysiol.1973.sp010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling J. E., Boycott B. B. Organization of the primate retina: electron microscopy. Proc R Soc Lond B Biol Sci. 1966 Nov 15;166(1002):80–111. doi: 10.1098/rspb.1966.0086. [DOI] [PubMed] [Google Scholar]
- Enroth-Cugell C., Robson J. G. The contrast sensitivity of retinal ganglion cells of the cat. J Physiol. 1966 Dec;187(3):517–552. doi: 10.1113/jphysiol.1966.sp008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukada Y. Receptive field organization of cat optic nerve fibers with special reference to conduction velocity. Vision Res. 1971 Mar;11(3):209–226. doi: 10.1016/0042-6989(71)90186-6. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond P. Chromatic sensitivity and spatial organization of LGN neurone receptive fields in cat: cone-rod interaction. J Physiol. 1972 Sep;225(2):391–413. doi: 10.1113/jphysiol.1972.sp009946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond P. Chromatic sensitivity and spatial organization of cat visual cortical cells: cone-rod interaction. J Physiol. 1971 Mar;213(2):475–494. doi: 10.1113/jphysiol.1971.sp009394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond P. Contrasts in spatial organization of receptive fields at geniculate and retinal levels: centre, surround and outer surround. J Physiol. 1973 Jan;228(1):115–137. doi: 10.1113/jphysiol.1973.sp010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond P., James C. R. The Purkinje shift in cat: extent of the mesopic range. J Physiol. 1971 Jul;216(1):99–109. doi: 10.1113/jphysiol.1971.sp009511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond P. Proceedings: Is retinal receptive field shape systematically related to retinal location? J Physiol. 1973 Oct;234(2):64P–66P. [PubMed] [Google Scholar]
- Honrubia F. M., Elliott J. H. Dendritic fields of the retinal ganglion cells in the cat. Arch Ophthalmol. 1970 Aug;84(2):221–226. doi: 10.1001/archopht.1970.00990040223016. [DOI] [PubMed] [Google Scholar]
- Ikeda H., Wright M. J. Receptive field organization of 'sustained' and 'transient' retinal ganglion cells which subserve different function roles. J Physiol. 1972 Dec;227(3):769–800. doi: 10.1113/jphysiol.1972.sp010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOZAK W., RODIECK R. W., BISHOP P. O. RESPONSES OF SINGLE UNITS IN LATERAL GENICULATE NUCLEUS OF CAT TO MOVING VISUAL PATTERNS. J Neurophysiol. 1965 Jan;28:19–47. doi: 10.1152/jn.1965.28.1.19. [DOI] [PubMed] [Google Scholar]
- KUFFLER S. W. Discharge patterns and functional organization of mammalian retina. J Neurophysiol. 1953 Jan;16(1):37–68. doi: 10.1152/jn.1953.16.1.37. [DOI] [PubMed] [Google Scholar]
- Leicester J., Stone J. Ganglion, amacrine and horizontal cells of the cat's retina. Vision Res. 1967 Sep;7(9):695–705. doi: 10.1016/0042-6989(67)90033-8. [DOI] [PubMed] [Google Scholar]
- Ogawa T., Bishop P. O., Levick W. R. Temporal characteristics of responses to photic stimulation by single ganglion cells in the unopened eye of the cat. J Neurophysiol. 1966 Jan;29(1):1–30. doi: 10.1152/jn.1966.29.1.1. [DOI] [PubMed] [Google Scholar]
- Pettigrew J. D., Nikara T., Bishop P. O. Responses to moving slits by single units in cat striate cortex. Exp Brain Res. 1968;6(4):373–390. doi: 10.1007/BF00233185. [DOI] [PubMed] [Google Scholar]
- Rodieck R. W. Receptive fields in the cat retina: a new type. Science. 1967 Jul 7;157(3784):90–92. doi: 10.1126/science.157.3784.90. [DOI] [PubMed] [Google Scholar]
- Rodieck R. W., Stone J. Analysis of receptive fields of cat retinal ganglion cells. J Neurophysiol. 1965 Sep;28(5):832–849. doi: 10.1152/jn.1965.28.5.833. [DOI] [PubMed] [Google Scholar]
- Rodieck R. W., Stone J. Response of cat retinal ganglion cells to moving visual patterns. J Neurophysiol. 1965 Sep;28(5):819–832. doi: 10.1152/jn.1965.28.5.819. [DOI] [PubMed] [Google Scholar]
- Spinelli D. N. Visual receptive fields in the cat's retina: complications. Science. 1966 Jun 24;152(3730):1768–1768. doi: 10.1126/science.152.3730.1768. [DOI] [PubMed] [Google Scholar]
- Sterling P., Wickelgren B. G. Visual receptive fields in the superior colliculus of the cat. J Neurophysiol. 1969 Jan;32(1):1–15. doi: 10.1152/jn.1969.32.1.1. [DOI] [PubMed] [Google Scholar]
- Stone J. A quantitative analysis of the distribution of ganglion cells in the cat's retina. J Comp Neurol. 1965 Jun;124(3):337–352. doi: 10.1002/cne.901240305. [DOI] [PubMed] [Google Scholar]
- Stone J., Fabian M. Specialized receptive fields of the cat's retina. Science. 1966 May 27;152(3726):1277–1279. doi: 10.1126/science.152.3726.1277. [DOI] [PubMed] [Google Scholar]
- Straschill M., Hoffmann K. P. Functional aspects of localization in the cat's tectum opticum. Brain Res. 1969 Apr;13(2):274–283. doi: 10.1016/0006-8993(69)90287-x. [DOI] [PubMed] [Google Scholar]
- Wässle H. Optical quality of the cat eye. Vision Res. 1971 Sep;11(9):995–1006. doi: 10.1016/0042-6989(71)90219-7. [DOI] [PubMed] [Google Scholar]


