Abstract
1. Cell membrane potential and input resistance measurements were made on segments of submaxillary glands from mice, rabbits or cats placed in a tissue bath, which was perfused with physiological salt solutions.
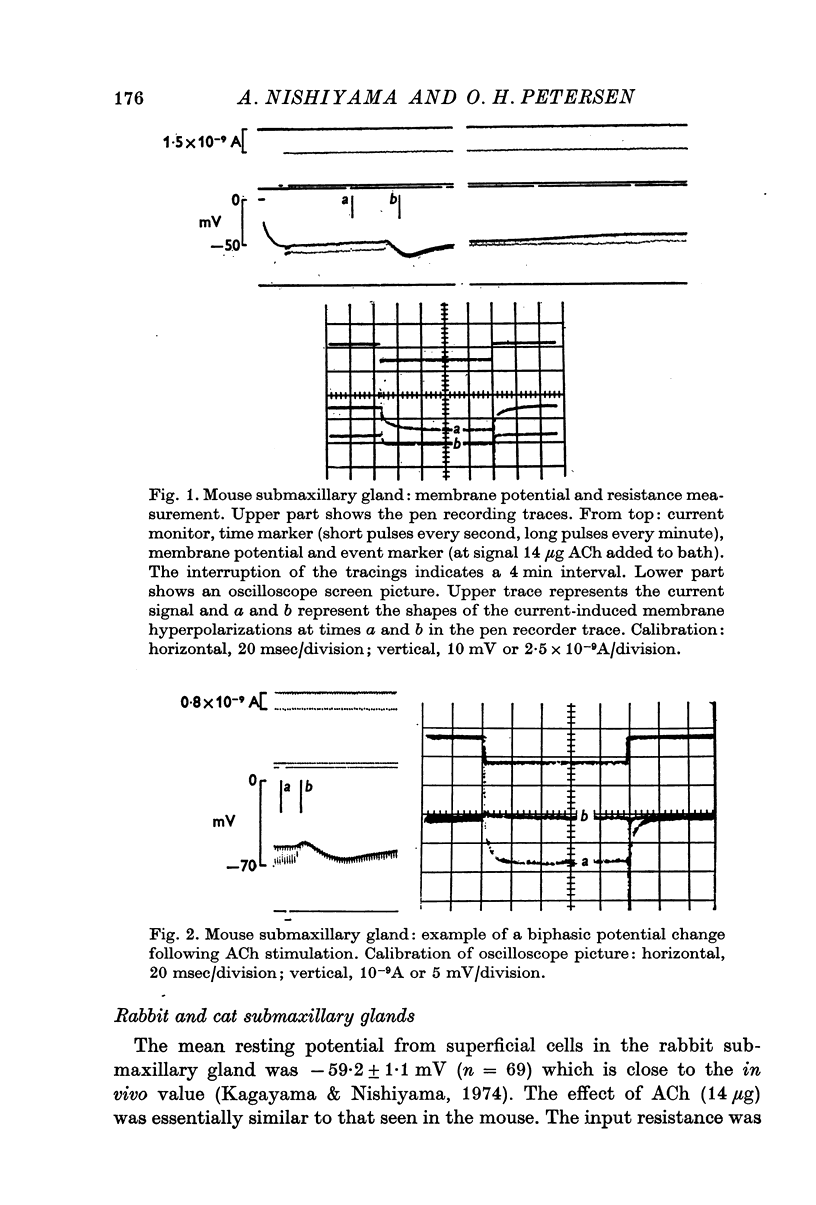
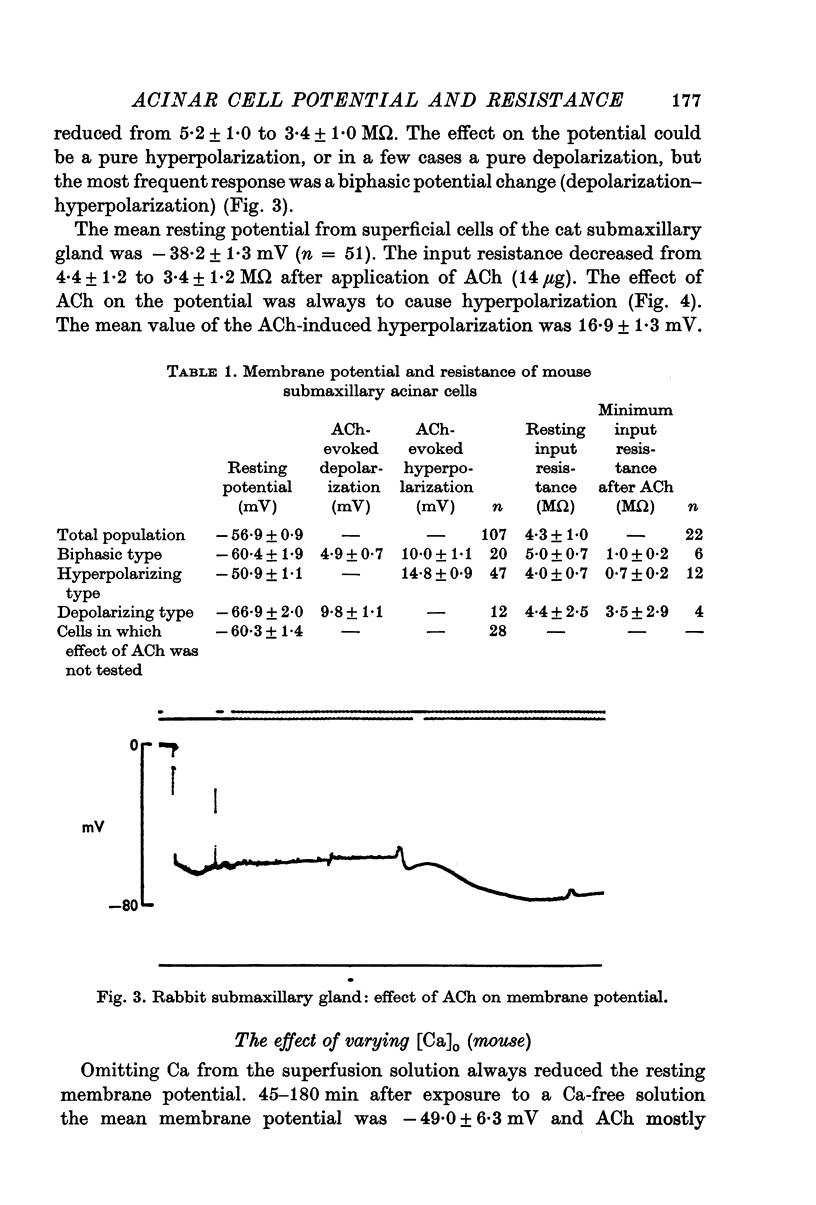
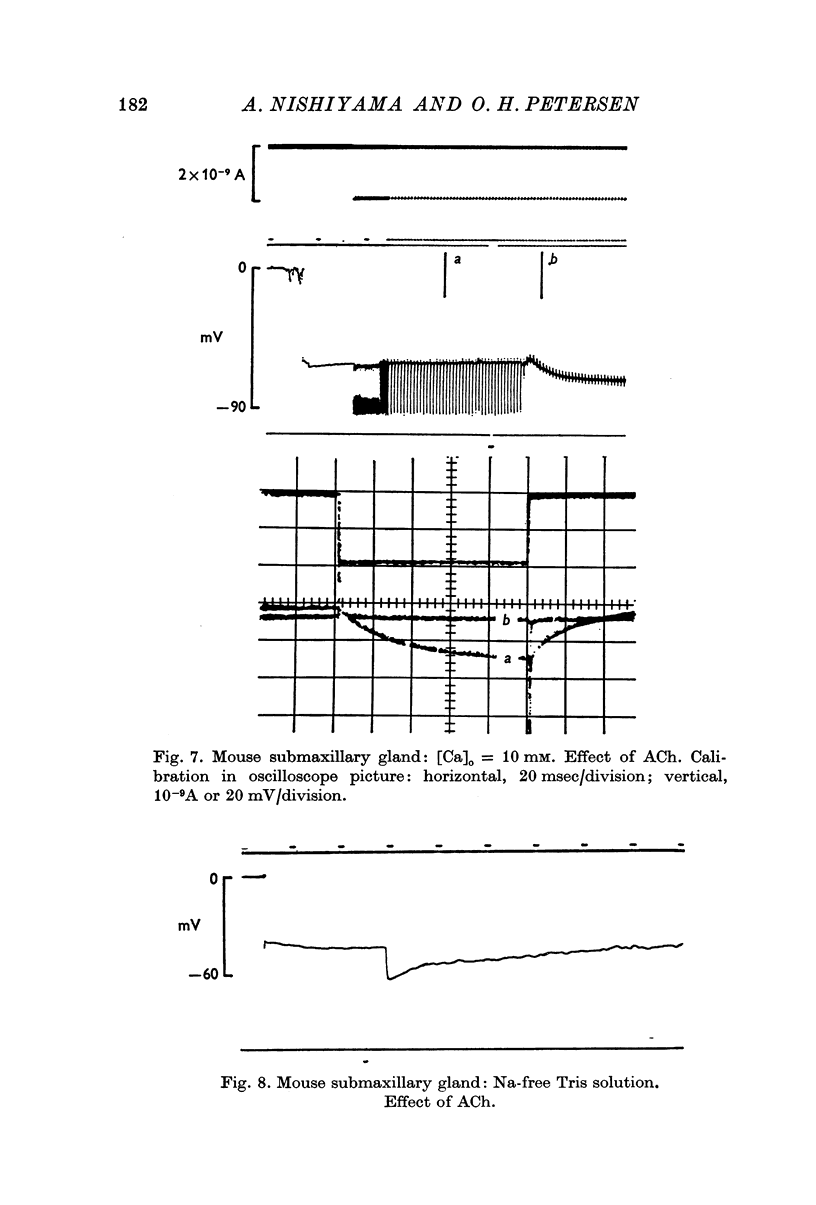
2. During exposure to a standard Krebs—Henseleit solution, ACh stimulation always evoked a marked decrease in input resistance and time constant. The change in potential evoked by ACh stimulation was either a monophasic hyperpolarization (low resting potential) or a depolarization followed by hyperpolarization (high resting potential).
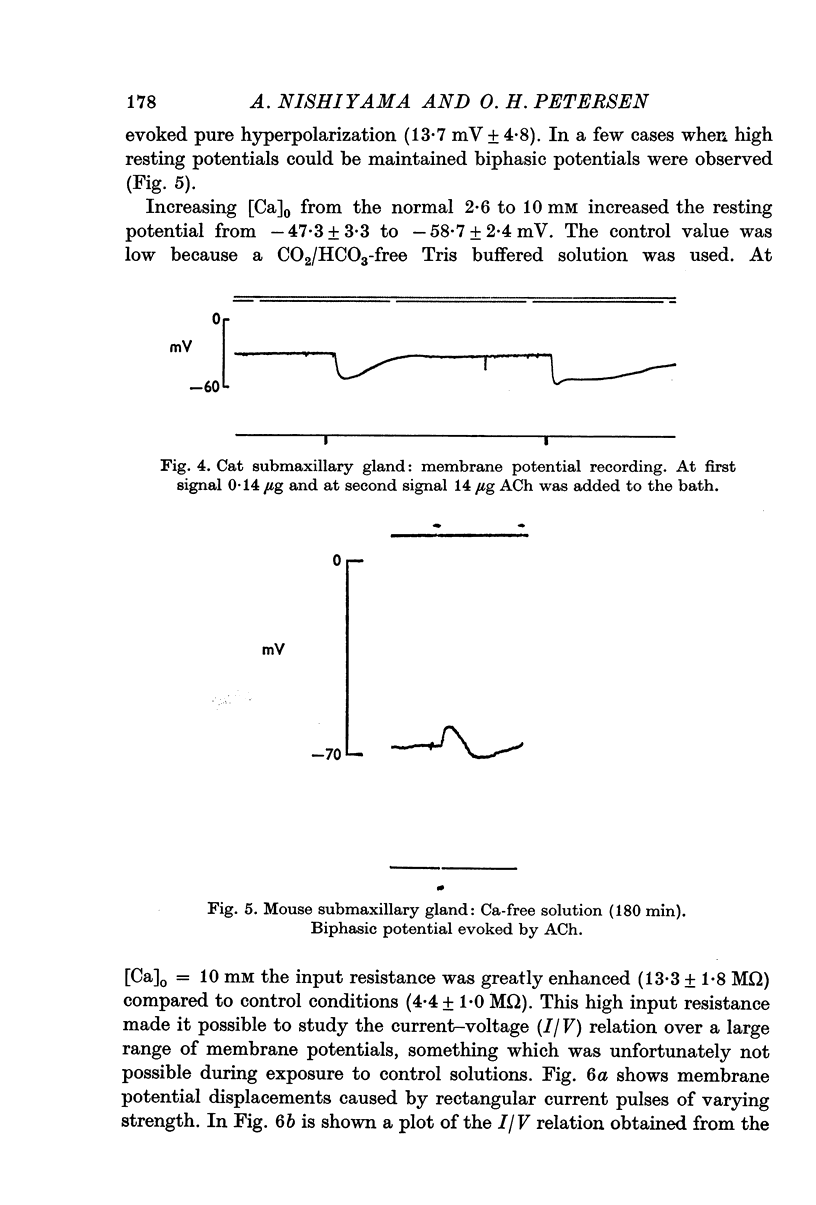
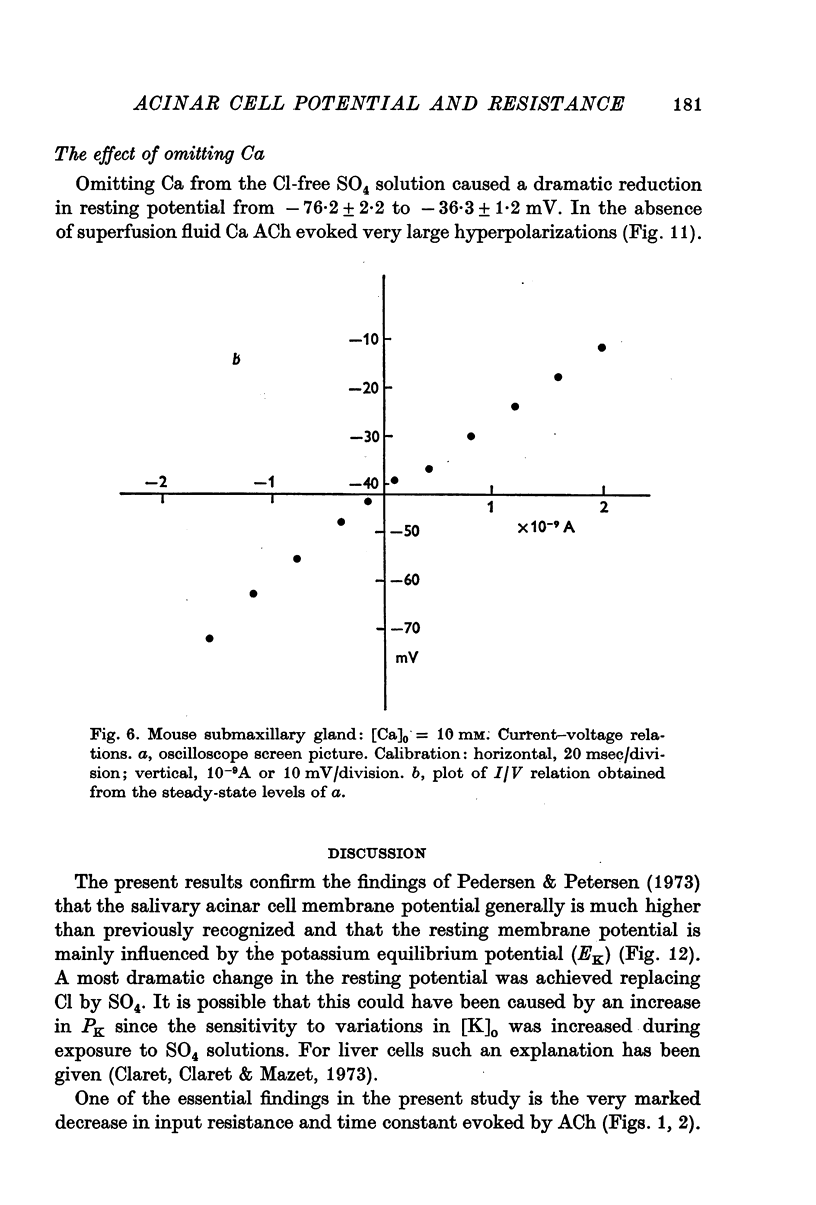
3. Increasing [Ca]o from 2·56 to 10 mM resulted in an enhanced input resistance. Under this condition it was sometimes possible to obtain current—voltage relations. The relationship was linear in the range -50 to -10 mV. In the absence of extracellular Ca the resting potential was reduced and ACh mostly evoked hyperpolarizations. In those cases when the resting potential remained high biphasic potentials were still observed.
4. During exposure to Na-free solutions the resting potential was either unchanged or slightly enhanced. ACh never evoked biphasic potentials, but always large hyperpolarizations.
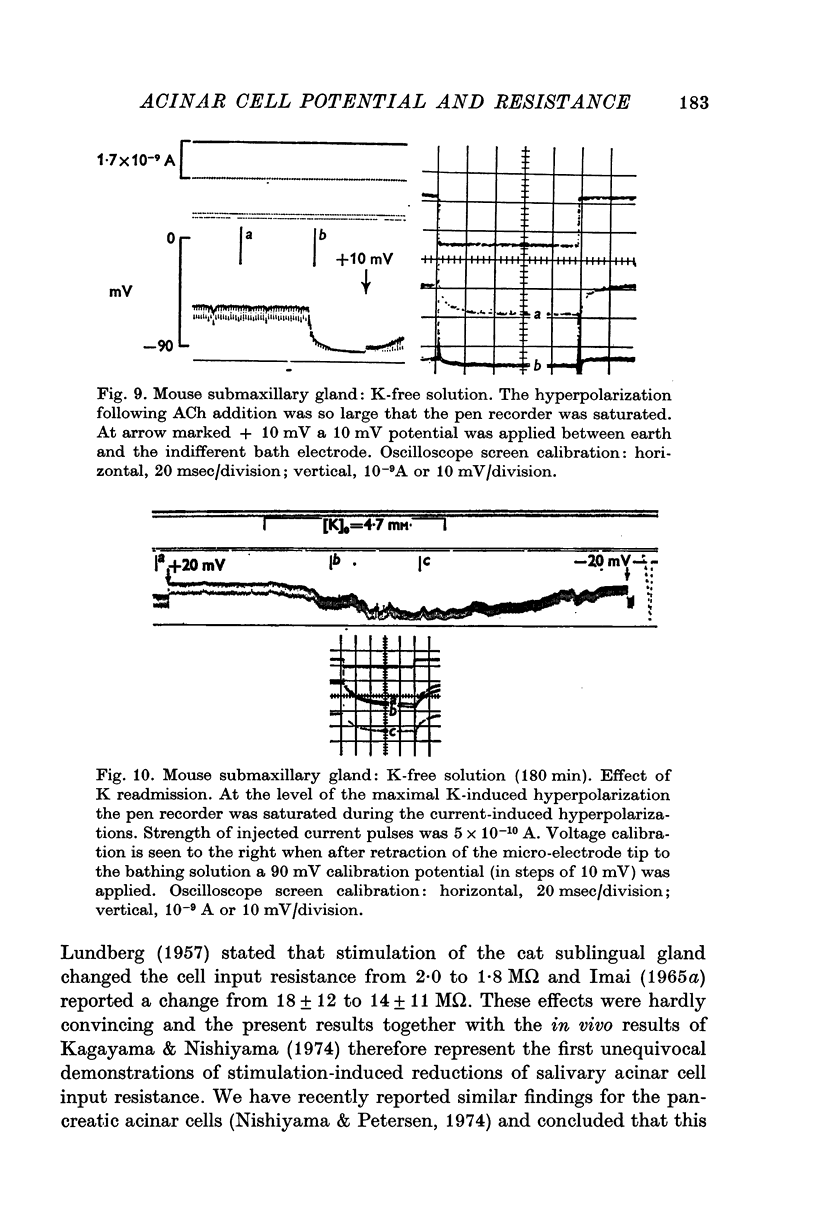
5. In the first period (1 hr) after exposure to a K-free solution ACh normally evoked very large hyperpolarizations, often to more than -100 mV. After several hours of exposure to K-free solution the input resistance gradually increased and ACh evoked a tremendous fall in input resistance and time constant with only a small potential change. Re-introducing control solution, ([K]o = 4·7) for a short period at this stage, caused a very marked hyperpolarization (about 30 mV) unaccompanied by a change in input resistance and time constant.
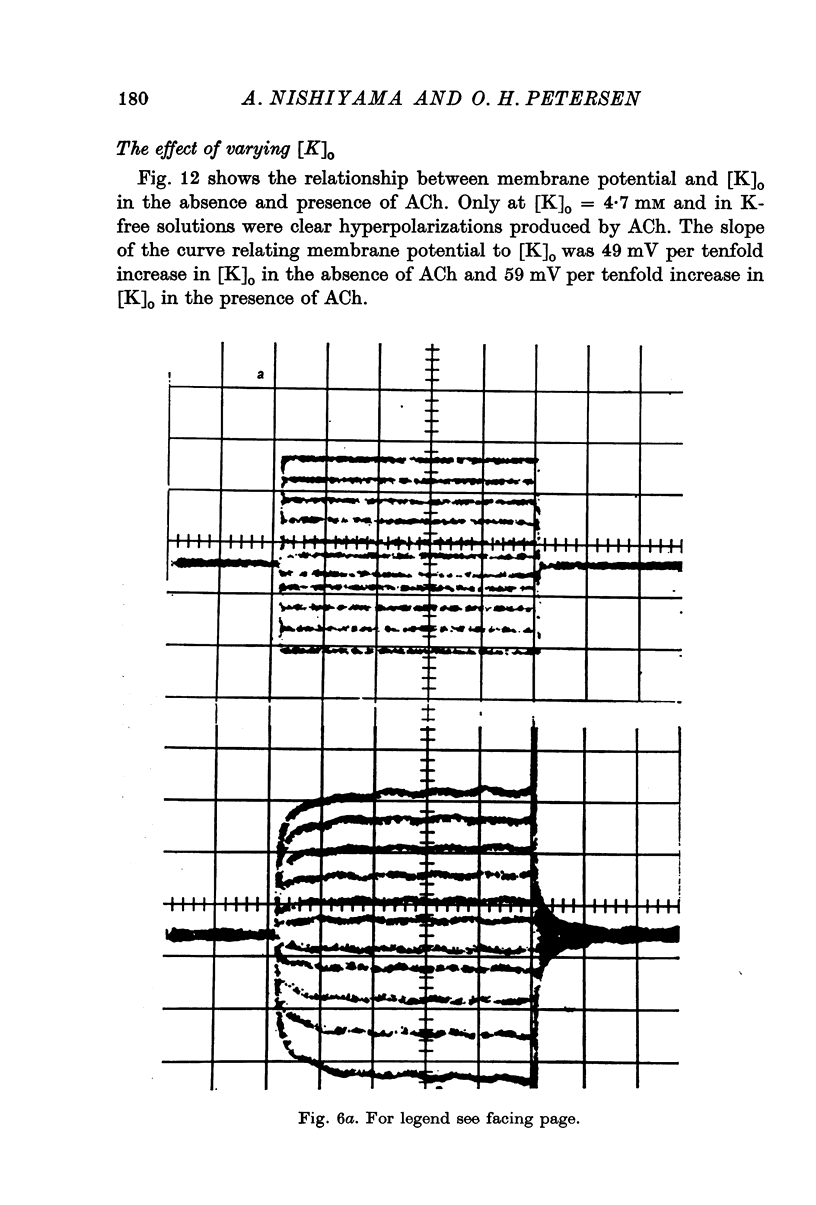
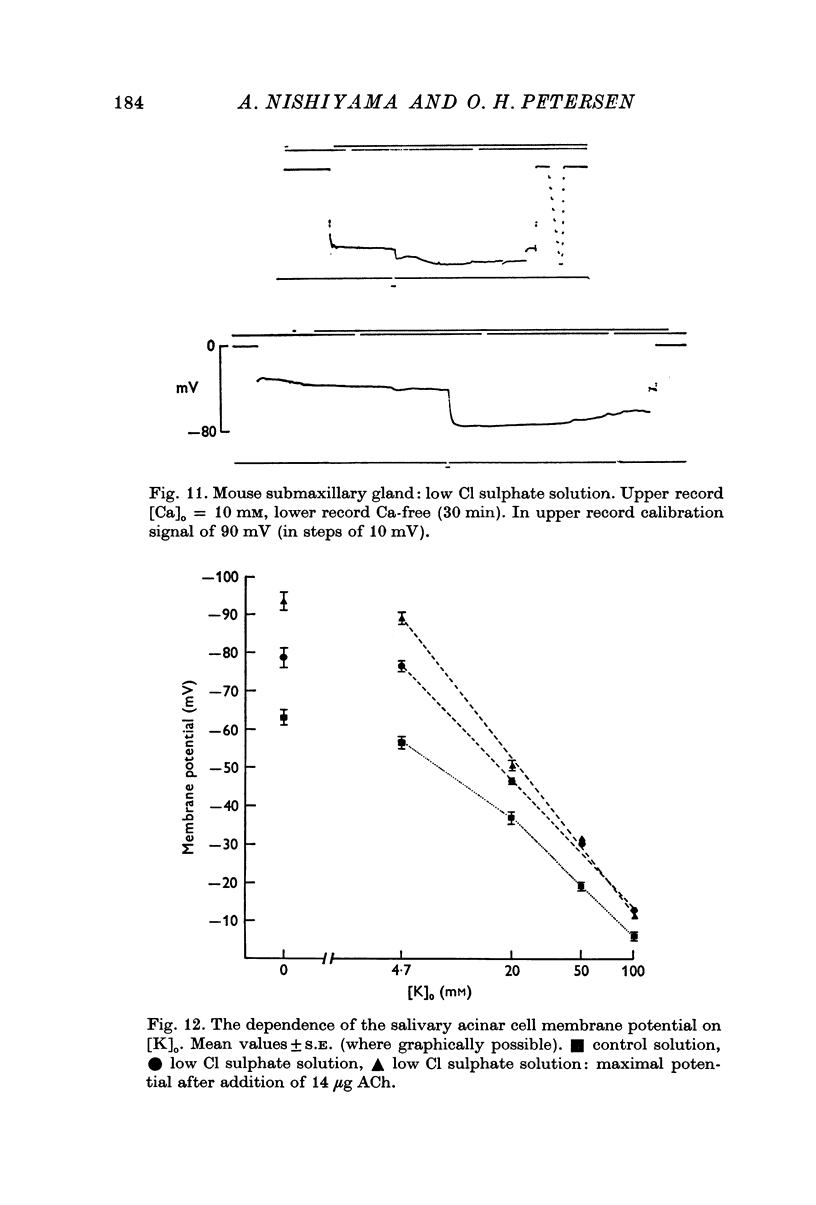
6. Replacing extracellular Cl by SO4 hyperpolarized the cell membrane. ACh mostly evoked hyperpolarization under this condition, but occasionally biphasic potentials were observed. Increasing [K] of the sulphate solution depolarized the cell membrane by about 49 mV per tenfold increase in [K]. In the presence of ACh the membrane behaved as a K-selective membrane with a slope of the linear curve relating membrane potential to [K]o of 59 mV per tenfold increase in [K]o.
7. It is concluded that ACh evokes a marked increase in surface cell membrane permeability of salivary acinar cells. The ACh evoked hyperpolarization is due to an increase in PK: the depolarization frequently preceding the hyperpolarization is probably mainly related to an increase in PNa. The membrane Na—K pump can act electrogenically at least under conditions of Na loading.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolton T. B. The role of electrogenic sodium pumping in the response of smooth muscle to acetylcholine. J Physiol. 1973 Feb;228(3):713–731. doi: 10.1113/jphysiol.1973.sp010108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claret B., Claret M., Mazet J. L. Ionic transport and membrane potential of rat liver cells in normal and low-chloride solutions. J Physiol. 1973 Apr;230(1):87–101. doi: 10.1113/jphysiol.1973.sp010176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean P. M., Matthews E. K. Pancreatic acinar cells: measurement of membrane potential and miniature depolarization potentials. J Physiol. 1972 Aug;225(1):1–13. doi: 10.1113/jphysiol.1972.sp009926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haylett D. G., Jenkinson D. H. Effects of noradrenaline on potassium reflux, membrane potential and electrolyte levels in tissue slices prepared from guinea-pig liver. J Physiol. 1972 Sep;225(3):721–750. doi: 10.1113/jphysiol.1972.sp009966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYNES R. D. CHLORIDE IN THE SQUID GIANT AXON. J Physiol. 1963 Dec;169:690–705. doi: 10.1113/jphysiol.1963.sp007289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagayama M., Nishiyama A. Membrane potential and input resistance in acinar cells from cat and rabbit submaxillary glands in vivo: effects of autonomic nerve stimulation. J Physiol. 1974 Oct;242(1):157–172. doi: 10.1113/jphysiol.1974.sp010699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUNDBERG A. The electrophysiology of the submaxillary gland of the cat. Acta Physiol Scand. 1955 Dec 22;35(1):1–25. doi: 10.1111/j.1748-1716.1955.tb01258.x. [DOI] [PubMed] [Google Scholar]
- LUNDBERG A. The mechanism of establishment of secretory potentials in sublingual gland cells. Acta Physiol Scand. 1957 Sep 17;40(1):35–58. doi: 10.1111/j.1748-1716.1957.tb01476.x. [DOI] [PubMed] [Google Scholar]
- Nielsen S. P., Petersen O. H. Transport of calcium in the perfused submandibular gland of the cat. J Physiol. 1972 Jun;223(3):685–697. doi: 10.1113/jphysiol.1972.sp009869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A., Petersen O. H. Pancreatic acinar cells: membrane potential and resistance change evoked by acetylcholine. J Physiol. 1974 Apr;238(1):145–158. doi: 10.1113/jphysiol.1974.sp010515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen G. L., Petersen O. H. Membrane potential measurement in parotid acinar cells. J Physiol. 1973 Oct;234(1):217–227. doi: 10.1113/jphysiol.1973.sp010342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen O. H. Electrogenic sodium pump in pancreatic acinar cells. Proc R Soc Lond B Biol Sci. 1973 Aug 31;184(1074):115–119. doi: 10.1098/rspb.1973.0037. [DOI] [PubMed] [Google Scholar]
- Petersen O. H. Electrophysiological studies on gland cells. Experientia. 1974 Feb 15;30(2):130–134. doi: 10.1007/BF01927689. [DOI] [PubMed] [Google Scholar]
- Petersen O. H. Membrane potential measurement in mouse salivary gland cells. Experientia. 1973 Feb 15;29(2):160–161. doi: 10.1007/BF01945448. [DOI] [PubMed] [Google Scholar]
- Petersen O. H., Pedersen G. L. Membrane effects mediated by alpha-and beta-adrenoceptors in mouse parotid acinar cells. J Membr Biol. 1974;16(4):353–362. doi: 10.1007/BF01872423. [DOI] [PubMed] [Google Scholar]
- Petersen O. H. Some factors influencing stimulation-induced release of potassium from the cat submandibular gland to fluid perfused through the gland. J Physiol. 1970 Jun;208(2):431–447. doi: 10.1113/jphysiol.1970.sp009129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen O. H. The dependence of the transmembrane salivary secretory potential on the external potassium and sodium concentration. J Physiol. 1970 Sep;210(1):205–215. doi: 10.1113/jphysiol.1970.sp009204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero P. J., Whittam R. The control by internal calcium of membrane permeability to sodium and potassium. J Physiol. 1971 May;214(3):481–507. doi: 10.1113/jphysiol.1971.sp009445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneyer L. H., Young J. A., Schneyer C. A. Salivary secretion of electrolytes. Physiol Rev. 1972 Jul;52(3):720–777. doi: 10.1152/physrev.1972.52.3.720. [DOI] [PubMed] [Google Scholar]
- Selinger Z., Batzri S., Eimerl S., Schramm M. Calcium and energy requirements for K + release mediated by the epinephrine -receptor in rat parotid slices. J Biol Chem. 1973 Jan 10;248(1):369–372. [PubMed] [Google Scholar]
- Yoshimura H., Imai Y. Studies on the secretory potential of acinal cells of the dog submaxillary gland and its ionic dependency. Jpn J Physiol. 1967 Jun;17(3):280–293. doi: 10.2170/jjphysiol.17.280. [DOI] [PubMed] [Google Scholar]


