Abstract
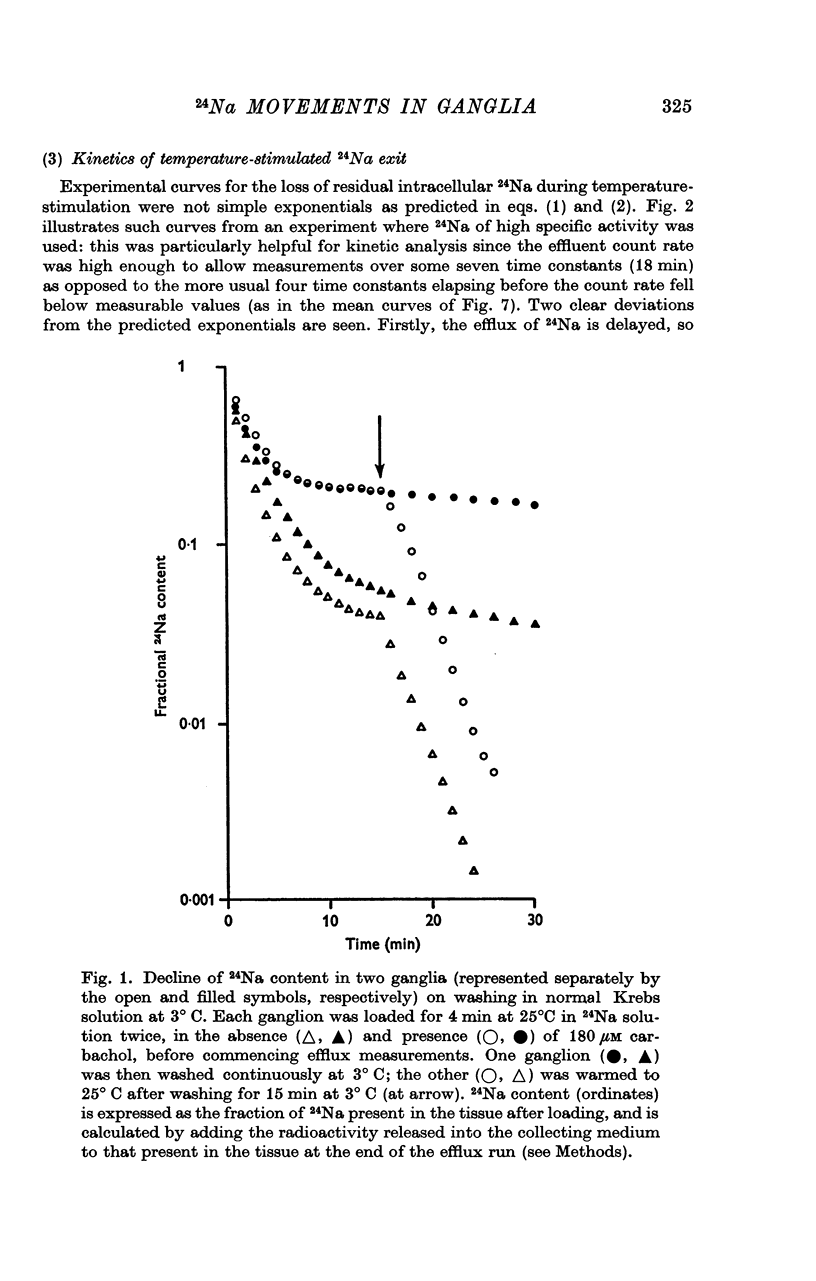
1. Isolated rat superior cervical ganglia were incubated in Krebs solution containing 24Na and carbachol for 4 min at 25° C. They were then washed at 3° C for 15 min to remove extracellular 24Na and the efflux of residual intracellular 24Na stimulated by warming to 25° C.
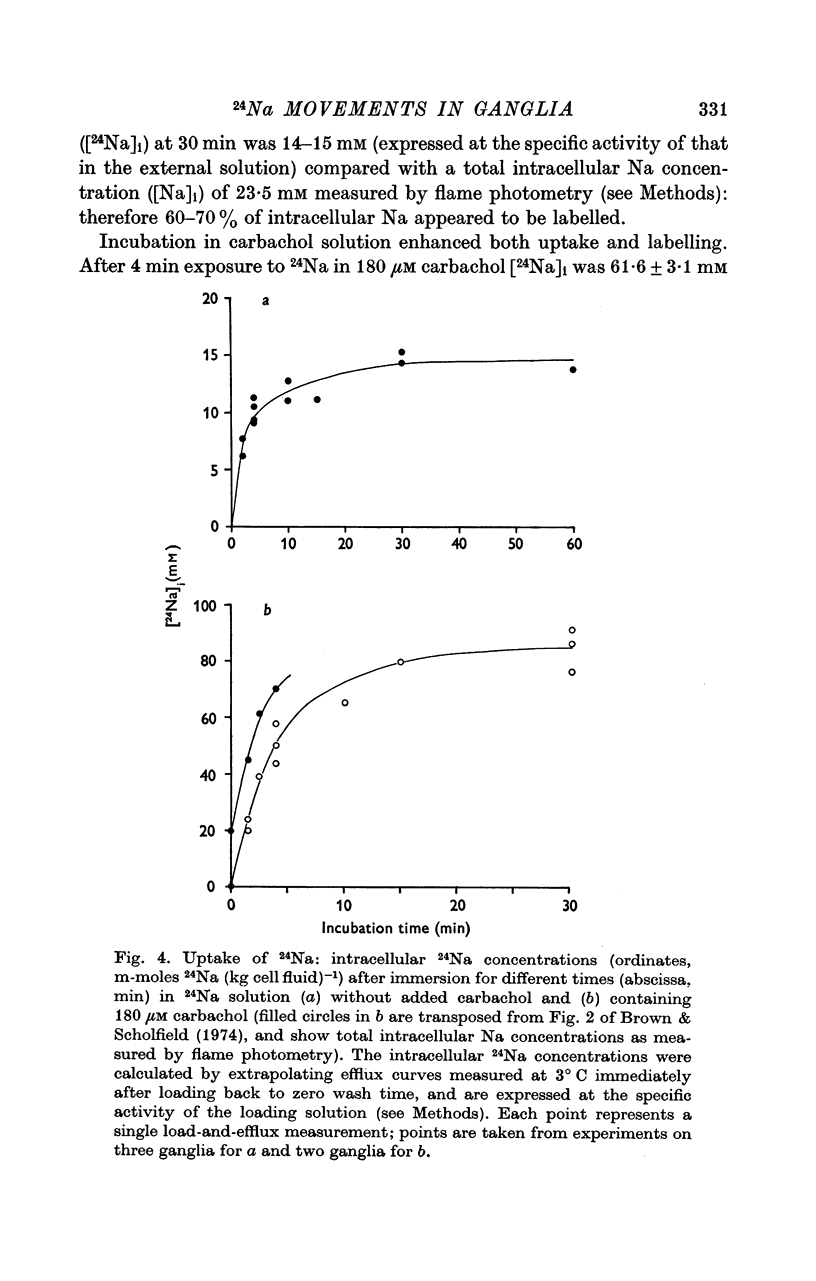
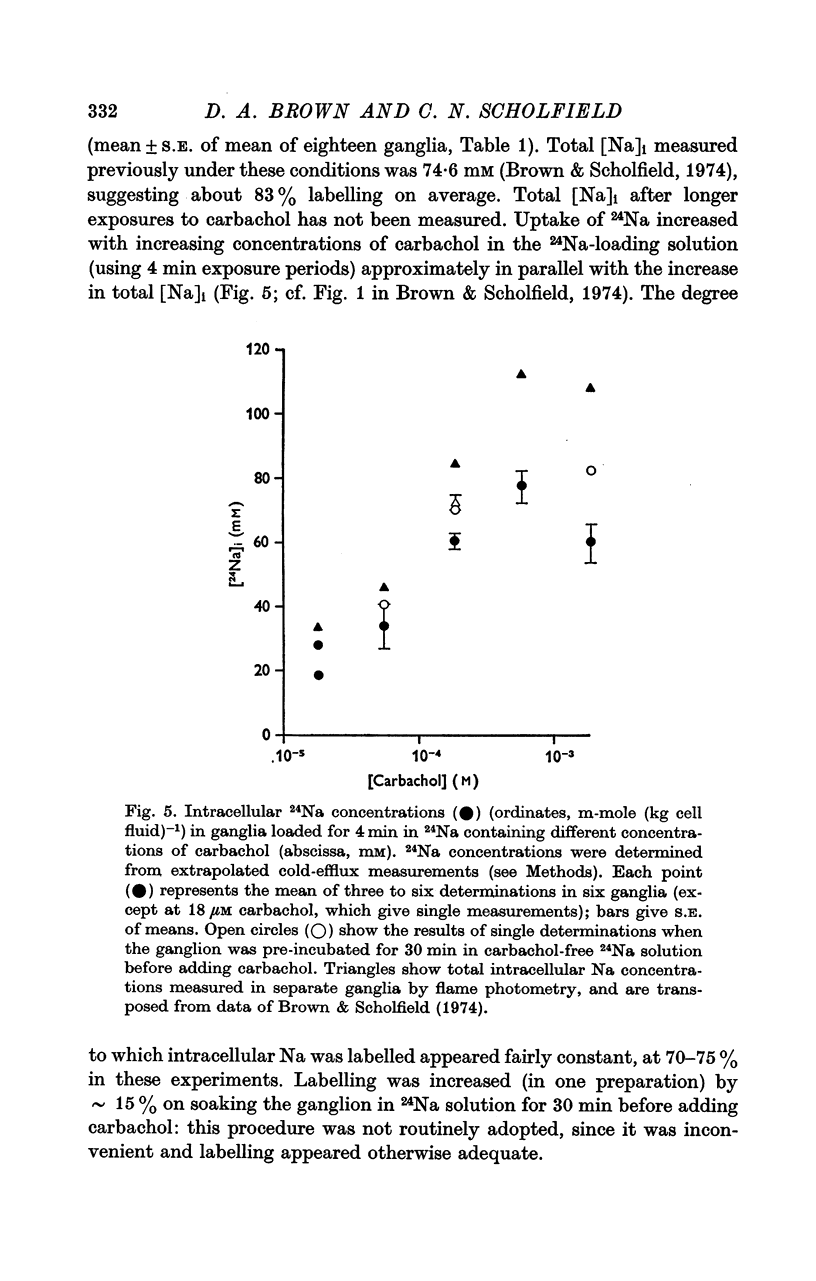
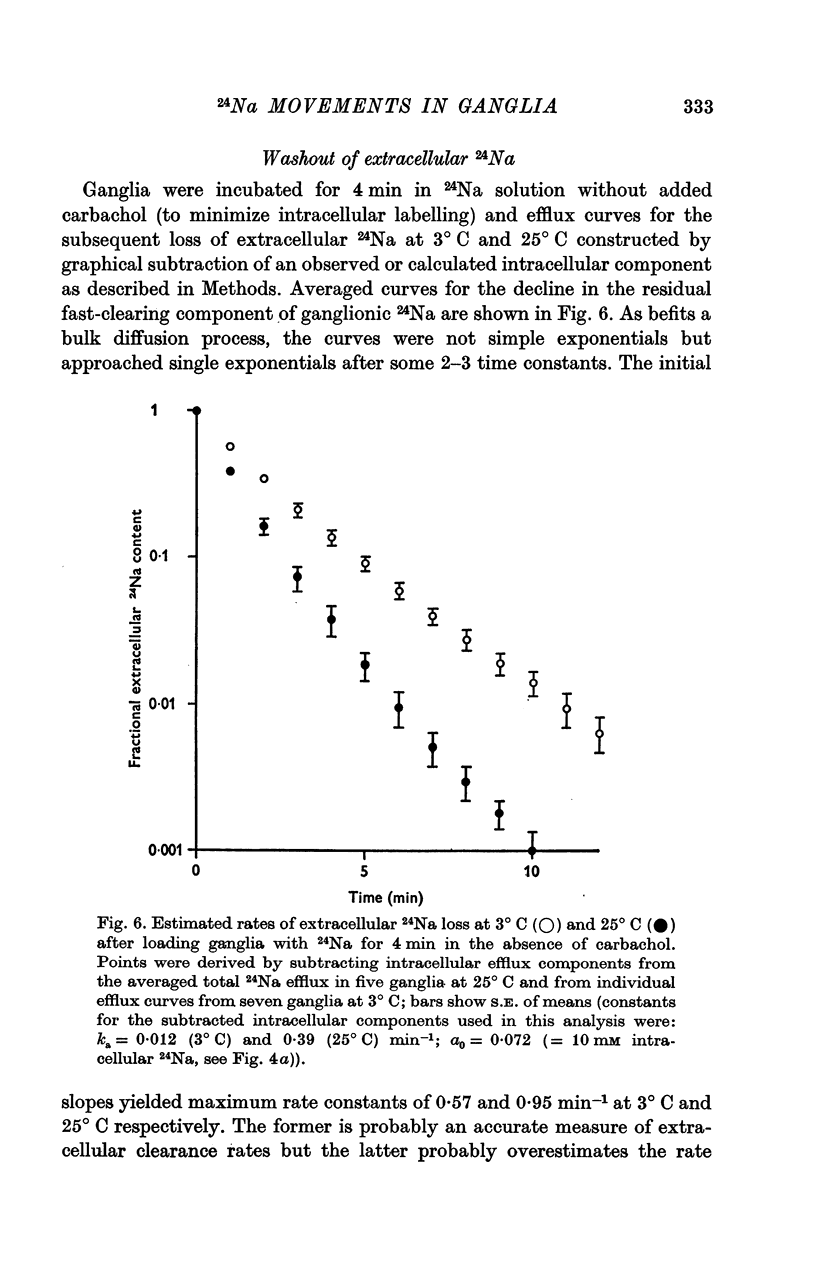
2. During the 15 min wash at 3° C desaturation curves became exponential with a rate constant of 0·012 ± 0·001 min-1 (n = 24). This was assumed to represent loss of intracellular 24Na, and initial uptake of 24Na was calculated therefrom by back-extrapolation to zero wash-time. After 4 min in 24Na + 180 μM carbachol intracellular [24Na] so calculated was 61·6 ± 3·1 mM (n = 18), representing 83% labelling of intracellular Na. In the absence of carbachol intracellular [24Na] was 10·0 ± 0·5 mM, representing 49% labelling. Extracellular Na was labelled by > 90% after 4 min in 24Na. The apparent rate constant for washout of extracellular 24Na was 0·6 min-1 at 3° C and 0·95 min-1 at 25° C.
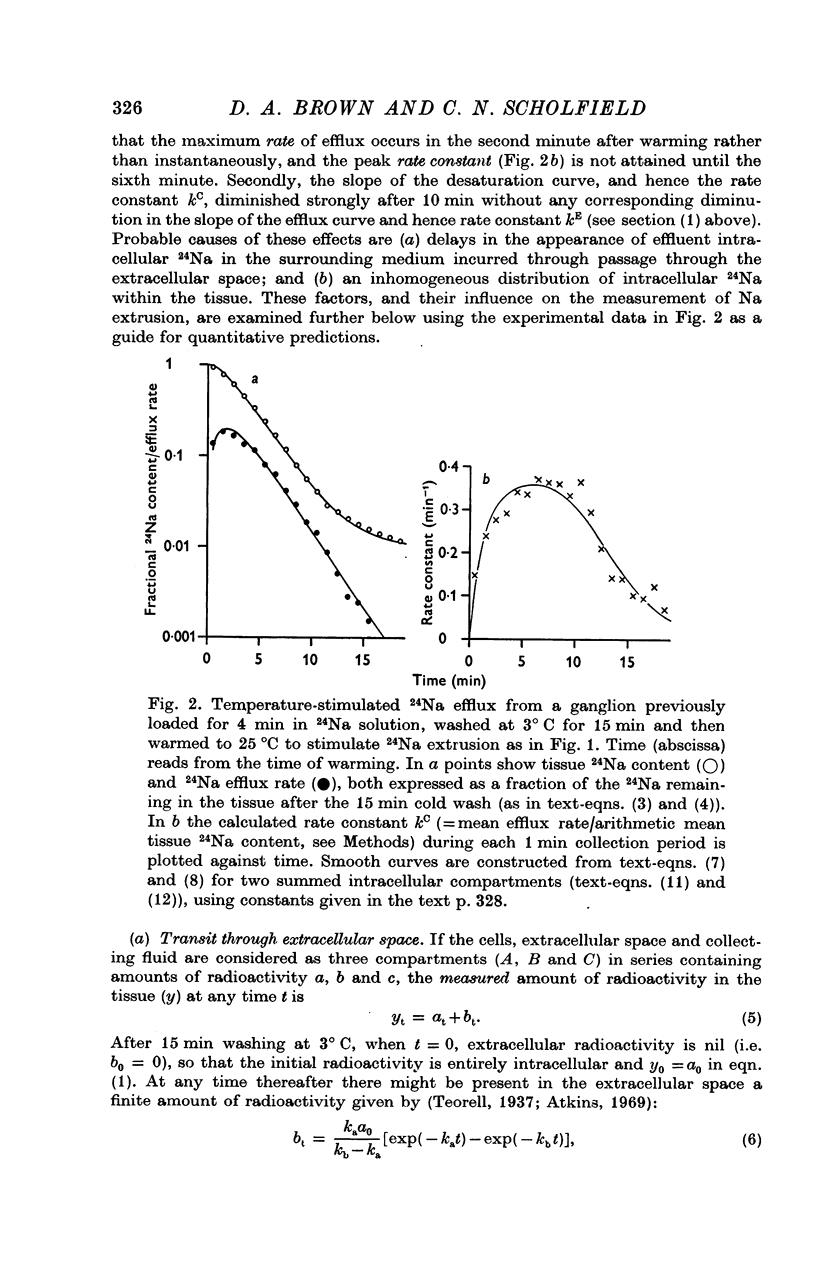
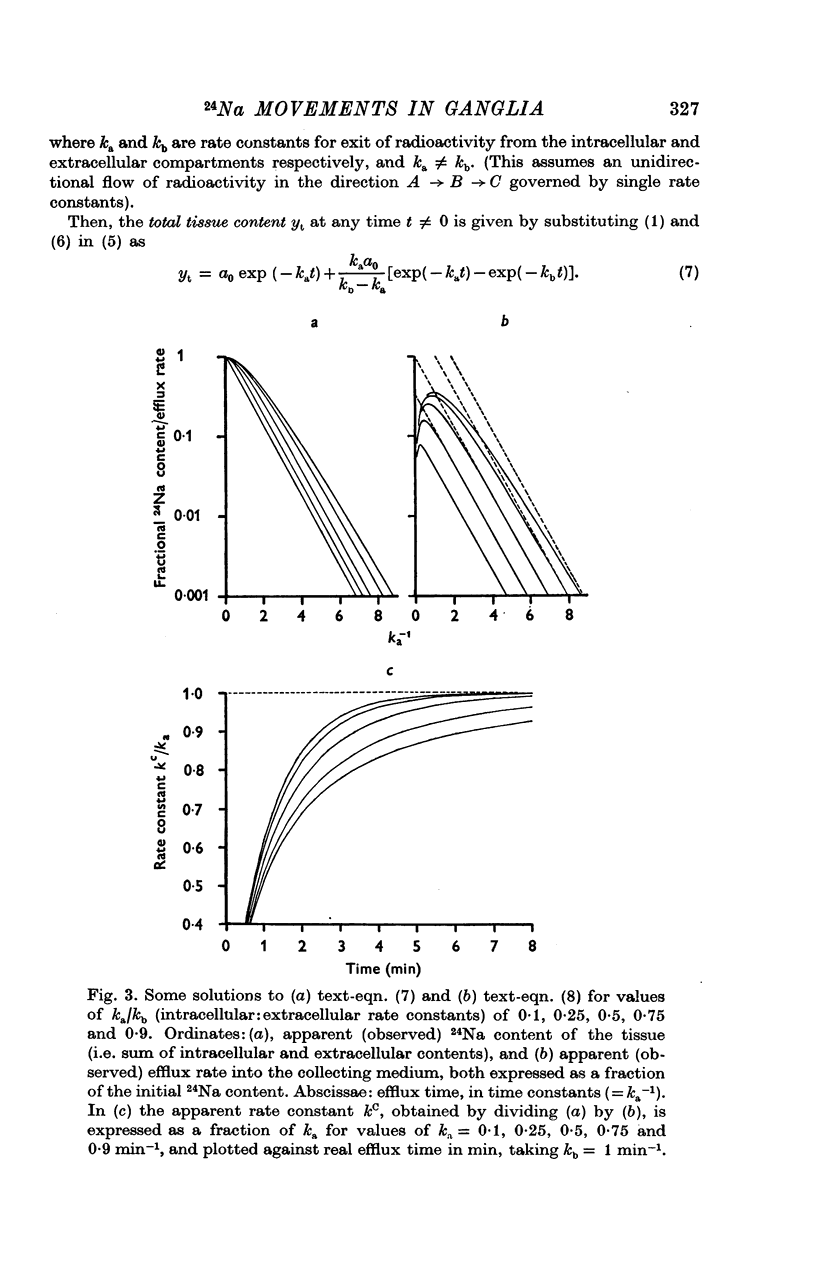
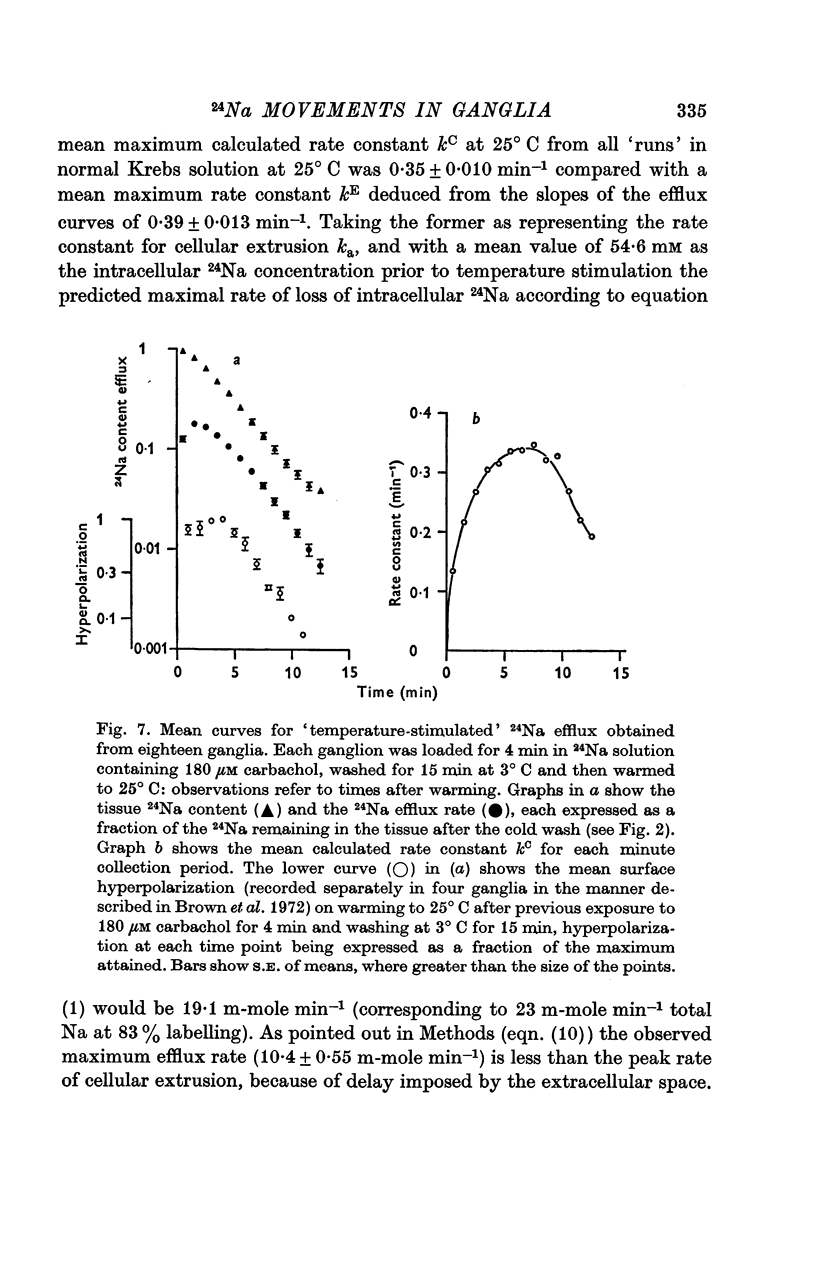
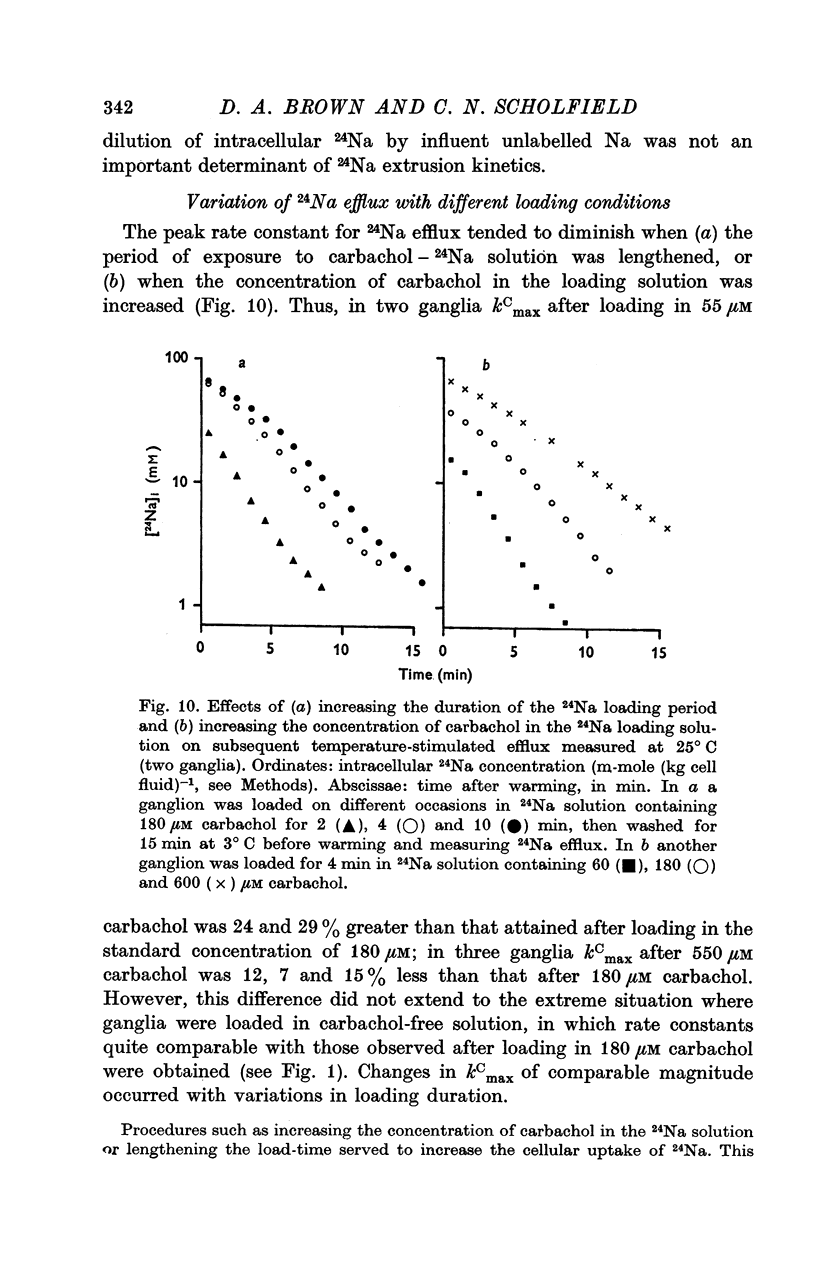
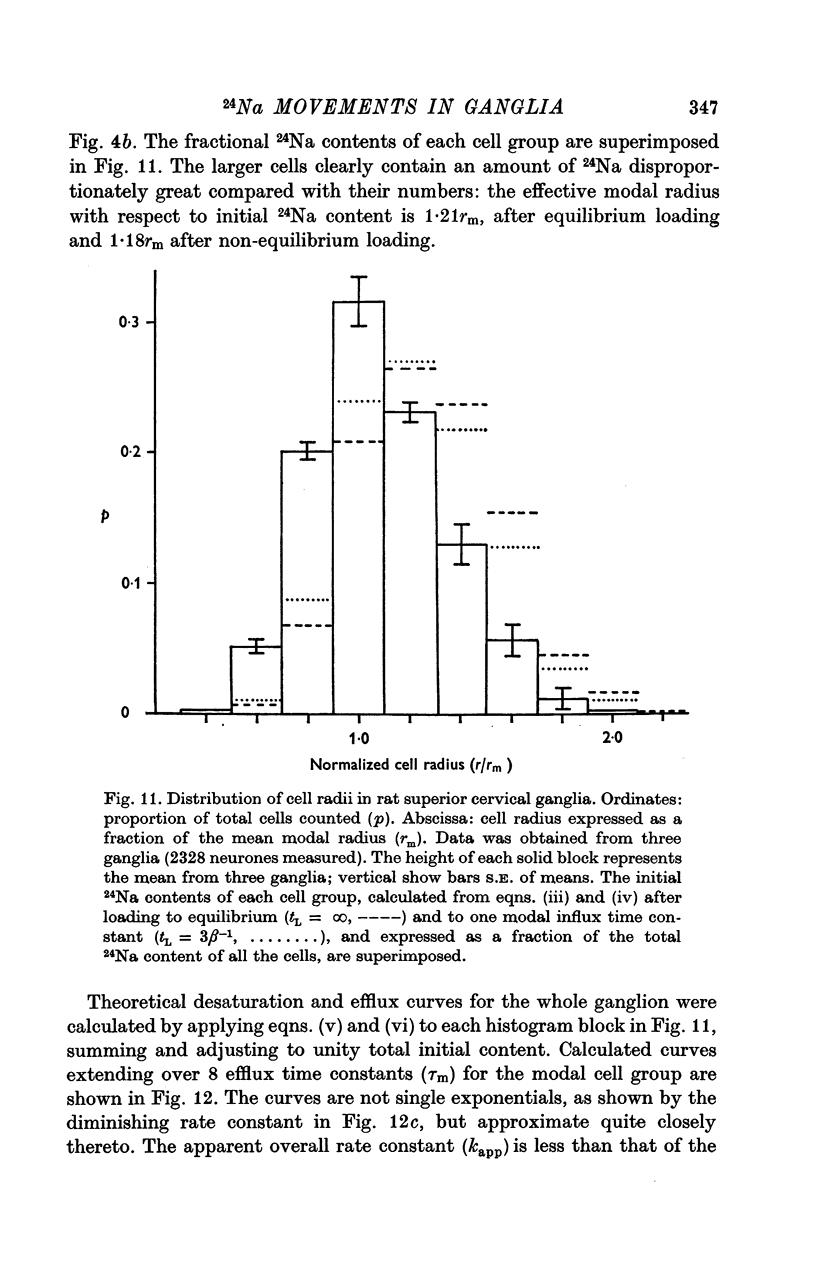
3. The loss of the residual intracellular 24Na during temperature stimulation was interpreted quantitatively in terms of an exponential decline of the bulk of intracellular 24Na with an extrusion rate constant of 0·39 ± 0·1 min-1 (n = 18), efflux being delayed by passage through the extracellular space with an effective rate constant of 0·8-1·2 min-1.
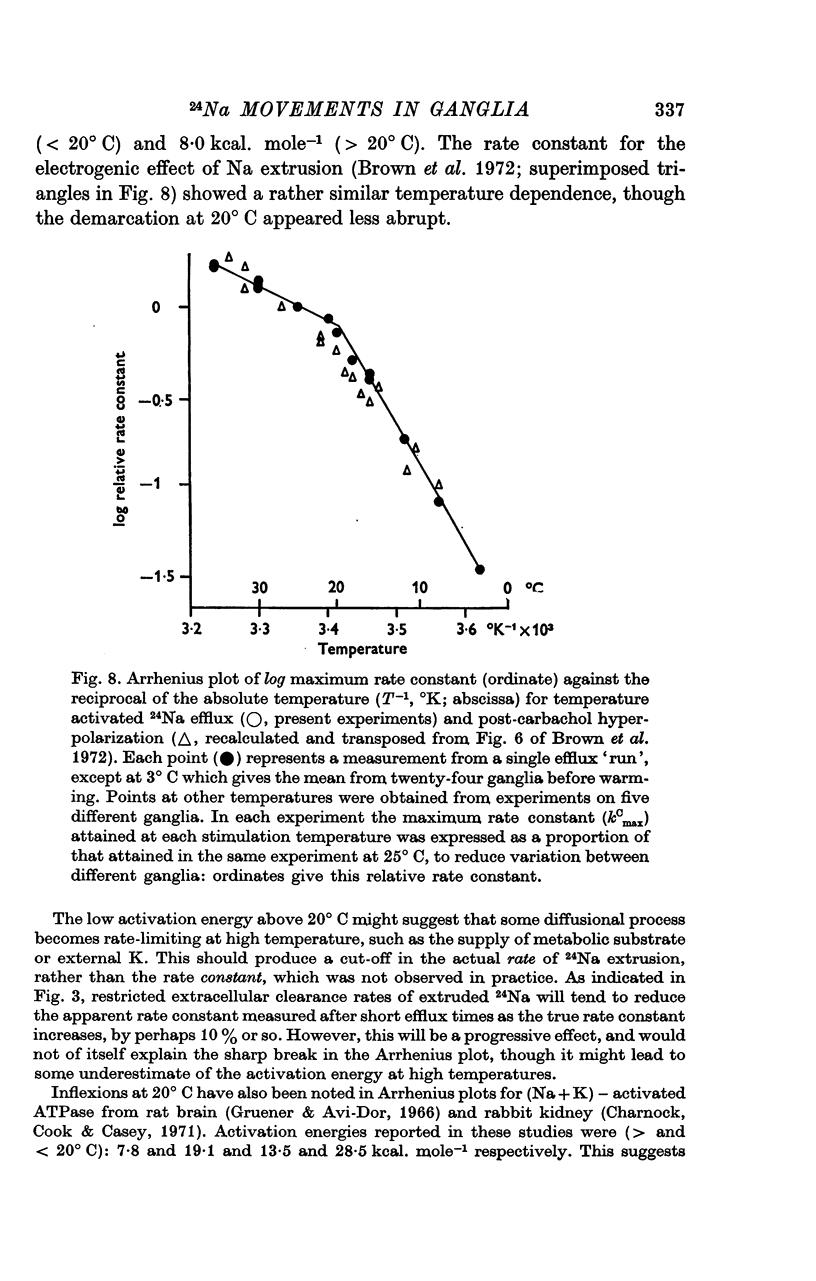
4. The peak rate constant (kC) for the desaturation curve at 25° C was 0·35 ± 0·01 min-1. An Arrhenius plot of log kC/T° K-1 yielded a two-stage linear regression with a transition at 20° C. Activation energies of 8 and 31 kcal. mole-1 were calculated above and below this transition respectively.
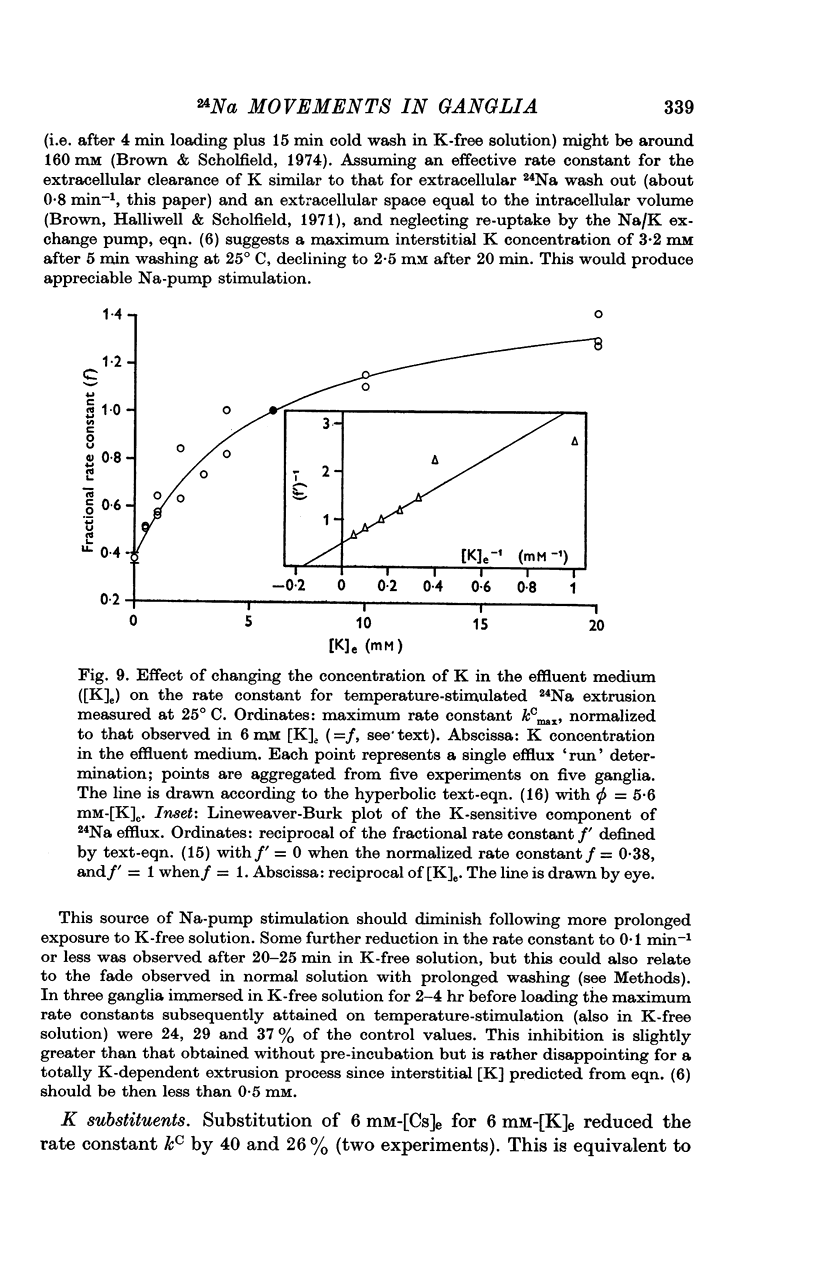
5. Omission of K from the 25° C temperature-stimulating solution reduced kC by 62%. The K-sensitive component of extrusion rate constant was a hyperbolic function of [K]e with half-saturation at 5·6 mM-[K]e and maximum kC of 0·58 min-1.
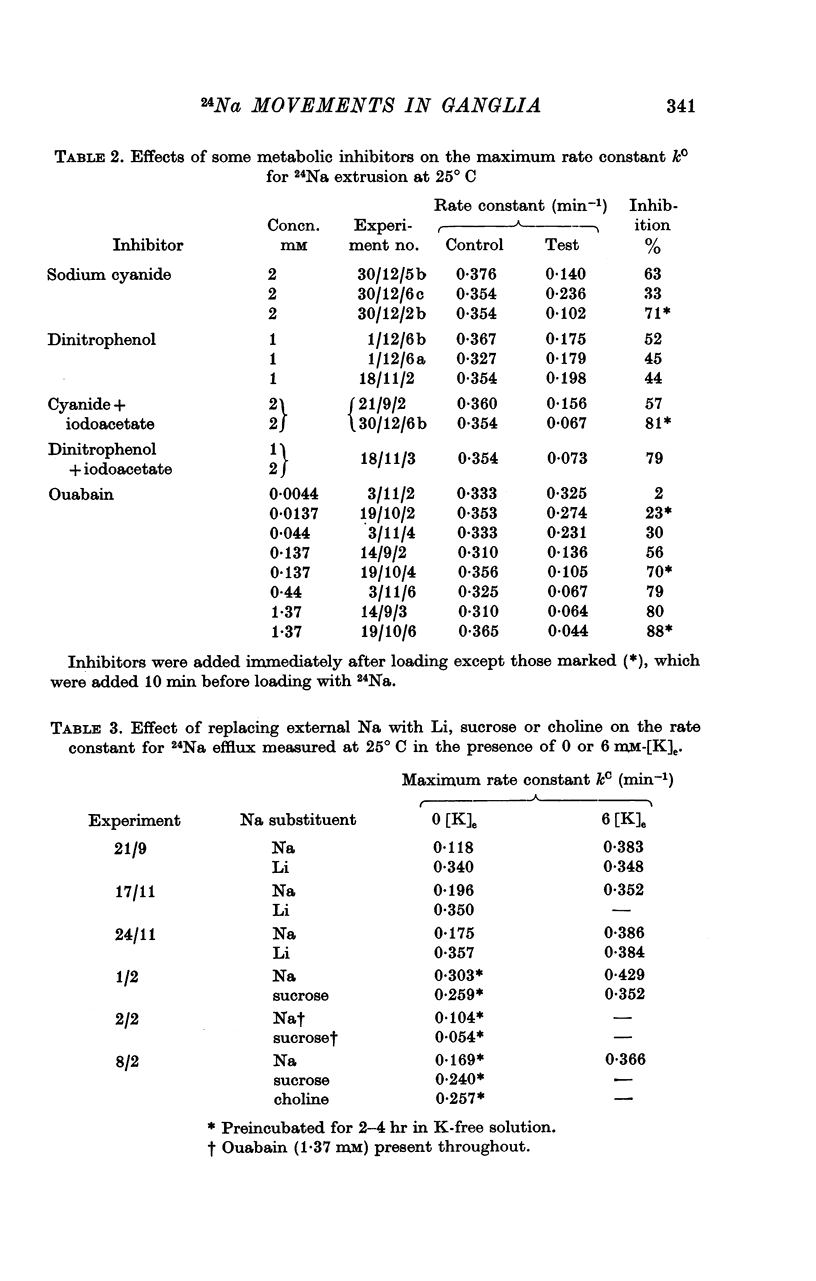
6. Cyanide (2 mM), 2,4-dinitrophenol (1 mM) and ouabain (1·4 mM) reduced kC by 50-90%. The half-maximally inhibiting concentration of ouabain was about 60 μM.
7. Substitution of sucrose, Li or choline for external Na did not reduce the extrusion rate of 24Na in either 6 mM-[K]e or 0 mM-[K]e. Li stimulated 24Na extrusion in Na-free, K-free solution.
8. The properties of the ganglionic Na pump deduced from rates of temperature-stimulated 24Na extrusion accord with the view that the ganglion hyperpolarization observed after Na loading by exposure to nicotinic depolarizing agents results from electrogenic Na extrusion. A comparable hyperpolarization is observed after temperature stimulation following Na loading.
Full text
PDF

















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRINLEY F. J. ION FLUXES IN THE CENTRAL NERVOUS SYSTEM. Int Rev Neurobiol. 1963;5:183–242. doi: 10.1016/s0074-7742(08)60596-6. [DOI] [PubMed] [Google Scholar]
- Baker P. F., Connelly C. M. Some properties of the external activation site of the sodium pump in crab nerve. J Physiol. 1966 Jul;185(2):270–297. doi: 10.1113/jphysiol.1966.sp007987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Shaw T. I. A comparison of the phosphorus metabolism of intact squid nerve with that of the isolated axoplasm and sheath. J Physiol. 1965 Sep;180(2):424–438. doi: 10.1113/jphysiol.1965.sp007710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittar E. E. An investigation of Na efflux from the toad oocyte using cold shock as a technic. Experientia. 1971 Mar 15;27(3):268–270. doi: 10.1007/BF02138138. [DOI] [PubMed] [Google Scholar]
- Bosteels S., Carmeliet E. Estimation of intracellular Na concentration and transmembrane Na flux in cardiac Purkyne fibres. Pflugers Arch. 1972;336(1):35–47. doi: 10.1007/BF00589140. [DOI] [PubMed] [Google Scholar]
- Brinley F. J., Jr, Mullins L. J. Sodium fluxes in internally dialyzed squid axons. J Gen Physiol. 1968 Aug;52(2):181–211. doi: 10.1085/jgp.52.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinley F. J., Jr Potassium accumulation and transport in the rat sympathetic ganglion. J Neurophysiol. 1967 Nov;30(6):1531–1560. doi: 10.1152/jn.1967.30.6.1531. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Brownstein M. J., Scholfield C. N. Origin of the after-hyperpolarization that follows removal of depolarizing agents from the isolated superior cervical ganglion of the rat. Br J Pharmacol. 1972 Apr;44(4):651–671. doi: 10.1111/j.1476-5381.1972.tb07305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Halliwell J. V., Scholfield C. N. Uptake of nicotine and extracellular space markers by isolated rat ganglia in relation to receptor activation. Br J Pharmacol. 1971 May;42(1):100–113. doi: 10.1111/j.1476-5381.1971.tb07090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Scholfield C. N. Changes of intracellular sodium and potassium ion concentrations in isolated rat superior cervical ganglia induced by depolarizing agents. J Physiol. 1974 Oct;242(2):307–319. doi: 10.1113/jphysiol.1974.sp010709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnock J. S., Cook D. A., Casey R. The role of cations and other factors on the apparent energy of activation of (Na + + K + )-ATPase. Arch Biochem Biophys. 1971 Nov;147(1):323–329. doi: 10.1016/0003-9861(71)90340-7. [DOI] [PubMed] [Google Scholar]
- DOLIVO M., LARRABEE M. G. Metabolism of glucose and oxygen in a mammalian sympathetic ganglion at reduced temperature and varied pH. J Neurochem. 1958 Oct;3(1):72–88. doi: 10.1111/j.1471-4159.1958.tb12611.x. [DOI] [PubMed] [Google Scholar]
- Garay R. P., Garrahan P. J. The interaction of sodium and potassium with the sodium pump in red cells. J Physiol. 1973 Jun;231(2):297–325. doi: 10.1113/jphysiol.1973.sp010234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrahan P. J., Glynn I. M. The behaviour of the sodium pump in red cells in the absence of external potassium. J Physiol. 1967 Sep;192(1):159–174. doi: 10.1113/jphysiol.1967.sp008294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn I. M. Membrane adenosine triphosphatase and cation transport. Br Med Bull. 1968 May;24(2):165–169. doi: 10.1093/oxfordjournals.bmb.a070620. [DOI] [PubMed] [Google Scholar]
- Grisham C. M., Barnett R. E. The role of lipid-phase transitions in the regulation of the (sodium + potassium) adenosine triphosphatase. Biochemistry. 1973 Jul 3;12(14):2635–2637. doi: 10.1021/bi00738a013. [DOI] [PubMed] [Google Scholar]
- Gruener N., Avi-Dor Y. Temperature-dependence of activation and inhibition of rat-brain adenosine triphosphatase activated by sodium and potassium ions. Biochem J. 1966 Sep;100(3):762–767. doi: 10.1042/bj1000762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRIS E. J., McLENNAN H. Cation exchanges in sympathetic ganglia. J Physiol. 1953 Sep;121(3):629–637. doi: 10.1113/jphysiol.1953.sp004970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEESEY J. C., WALLGREN H. MOVEMENTS OF RADIOACTIVE SODIUM IN CEREBRAL-CORTEX SLICES IN RESPONSE TO ELECTRICAL STIMULATION. Biochem J. 1965 May;95:301–310. doi: 10.1042/bj0950301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYNES R. D., LEWIS P. R. The resting exchange of radioactive potassium in crab nerve. J Physiol. 1951 Mar;113(1):73–98. doi: 10.1113/jphysiol.1951.sp004557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYNES R. D., SWAN R. C. The effect of external sodium concentration on the sodium fluxes in frog skeletal muscle. J Physiol. 1959 Oct;147:591–625. doi: 10.1113/jphysiol.1959.sp006264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYNES R. D. The ionic fluxes in frog muscle. Proc R Soc Lond B Biol Sci. 1954 May 27;142(908):359–382. doi: 10.1098/rspb.1954.0030. [DOI] [PubMed] [Google Scholar]
- Koketsu K. Cholinergic synaptic potentials and the underlying ionic mechasims. Fed Proc. 1969 Jan-Feb;28(1):101–112. [PubMed] [Google Scholar]
- Kosterlitz H. W., Lees G. M., Wallis D. I. Resting and action potentials recorded by the sucrose-gap method in the superior cervical ganglion of the rabbit. J Physiol. 1968 Mar;195(1):39–53. doi: 10.1113/jphysiol.1968.sp008445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LARRABEE M. G. Oxygen consumption of excised sympathetic ganglia at rest and in activity. J Neurochem. 1958;2(2-3):81–101. doi: 10.1111/j.1471-4159.1958.tb12355.x. [DOI] [PubMed] [Google Scholar]
- PERSOFF D. A. A comparison of methods for measuring efflux of labelled potassium from contracting rabbit atria. J Physiol. 1960 Jul;152:354–366. doi: 10.1113/jphysiol.1960.sp006492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perri V., Sacchi O., Caella C. Electrical properties and synaptic connections of the sympathetic neurons in the rat and guinea-pig superior cervical ganglion. Pflugers Arch. 1970;314(1):40–54. doi: 10.1007/BF00587045. [DOI] [PubMed] [Google Scholar]
- Rang H. P., Ritchie J. M. On the electrogenic sodium pump in mammalian non-myelinated nerve fibres and its activation by various external cations. J Physiol. 1968 May;196(1):183–221. doi: 10.1113/jphysiol.1968.sp008502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C. Electrogenic sodium pump in nerve and muscle cells. Physiol Rev. 1972 Jul;52(3):563–594. doi: 10.1152/physrev.1972.52.3.563. [DOI] [PubMed] [Google Scholar]
- Thomas R. C. Membrane current and intracellular sodium changes in a snail neurone during extrusion of injected sodium. J Physiol. 1969 Apr;201(2):495–514. doi: 10.1113/jphysiol.1969.sp008769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USSING H. H. Transport of ions across cellular membranes. Physiol Rev. 1949 Apr;29(2):127–155. doi: 10.1152/physrev.1949.29.2.127. [DOI] [PubMed] [Google Scholar]
- WHITTAM R. The dependence of the respiration of brain cortex on active cation transport. Biochem J. 1962 Jan;82:205–212. doi: 10.1042/bj0820205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zierler K. L. Interpretation of tracer washout curves from a population of muscle fibers. J Gen Physiol. 1966 Jan;49(3):423–431. doi: 10.1085/jgp.49.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]


