Abstract
1. The mechanism of depolarization of squid axon membranes caused by grayanotoxin I has been studied by means of internal perfusion and voltage clamp techniques.
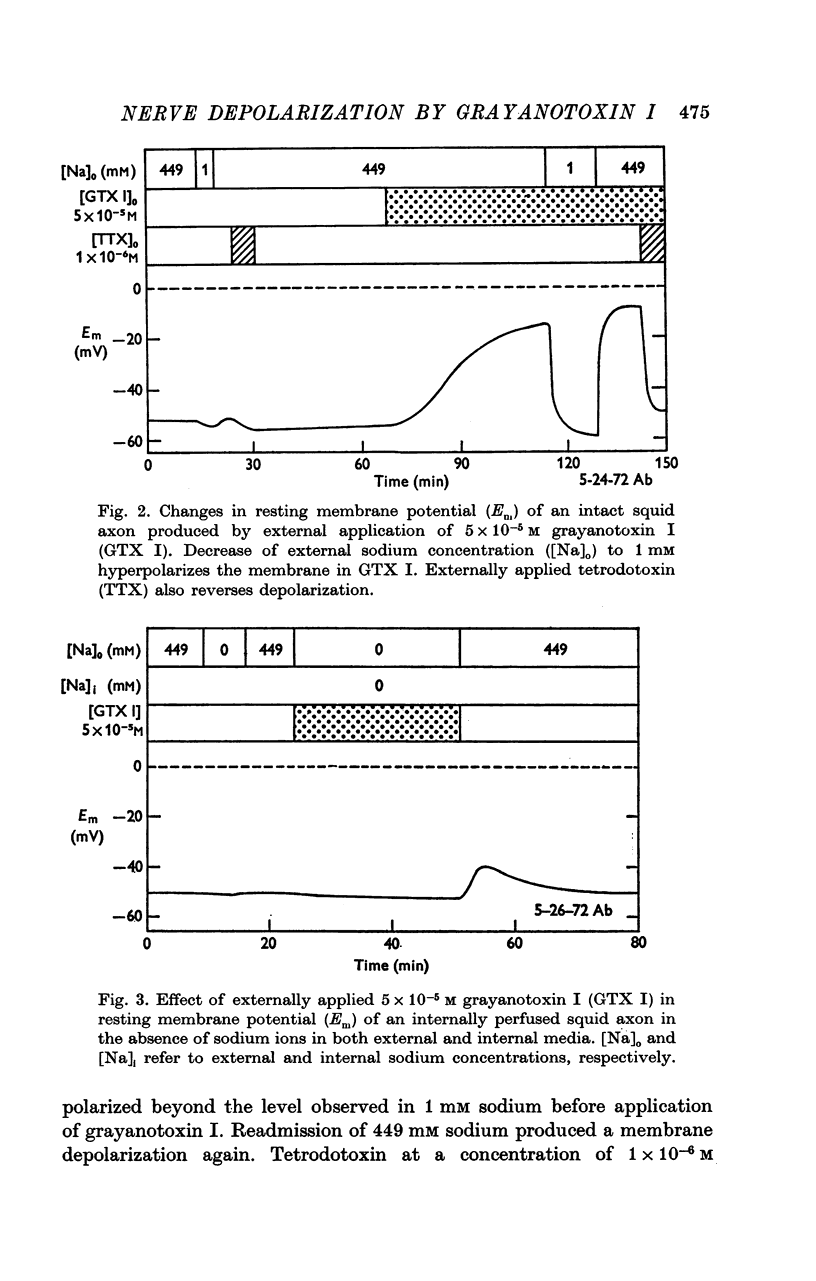
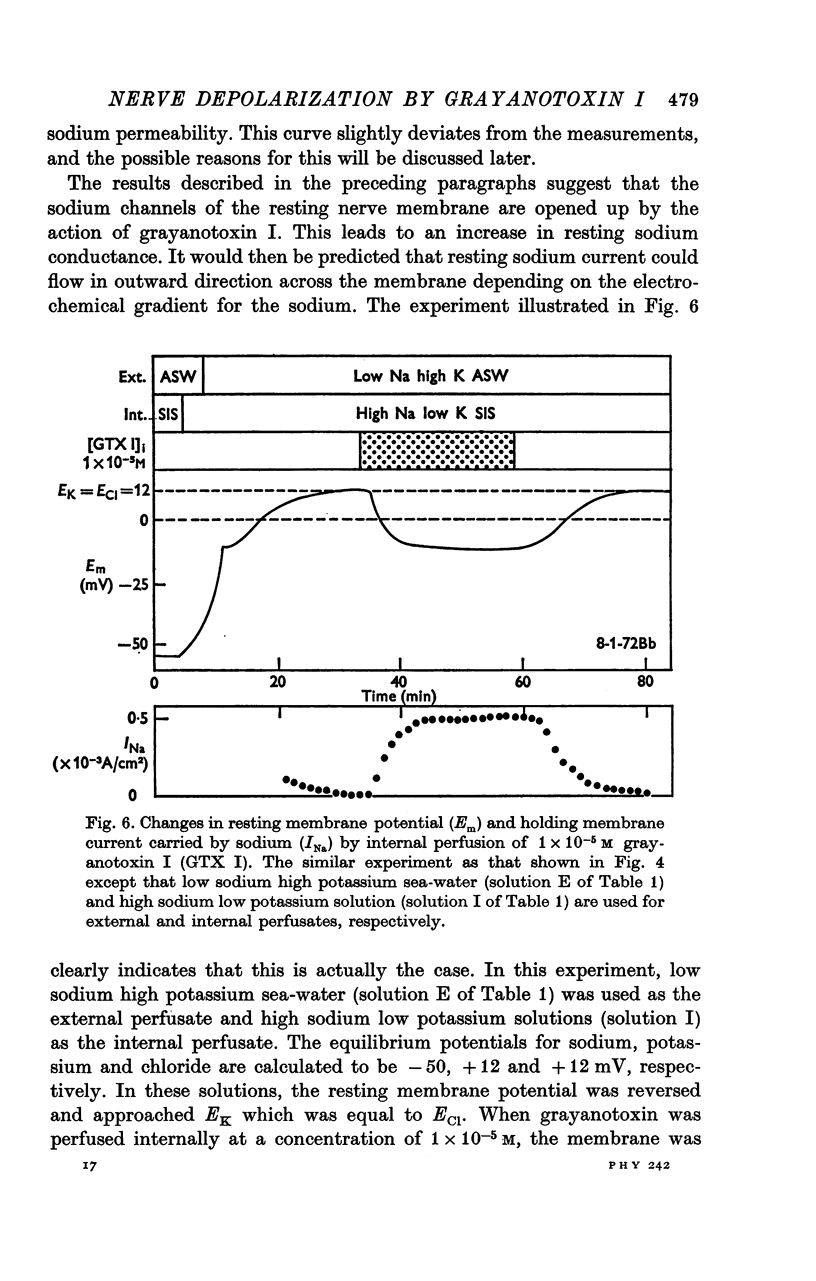
2. The depolarization induced by either internal or external application of grayanotoxin I was reversed by decreasing the external sodium concentration from 449 to 1 mm.
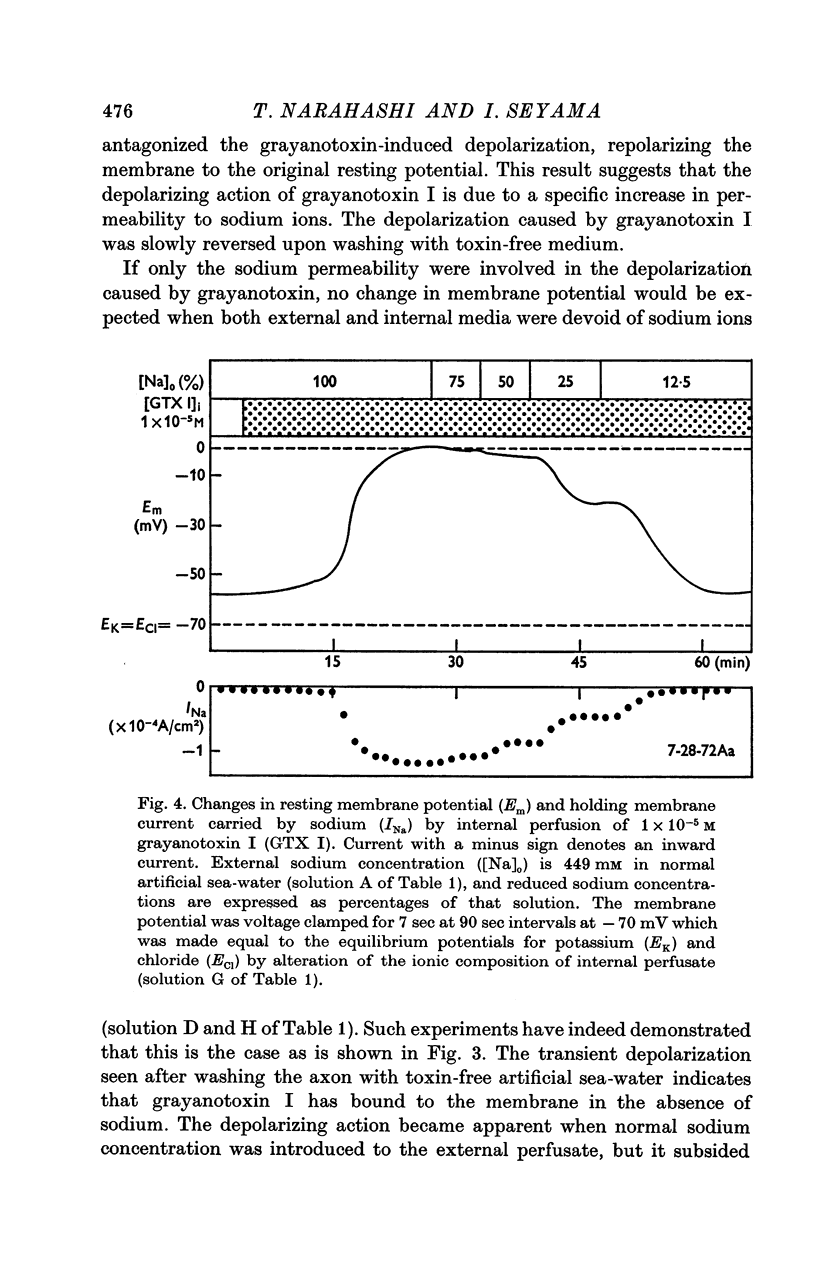
3. No depolarization was observed when both external and internal media were devoid of sodium ions, indicating that the depolarization by grayanotoxin I in normal media is due to a specific increase in resting sodium permeability.
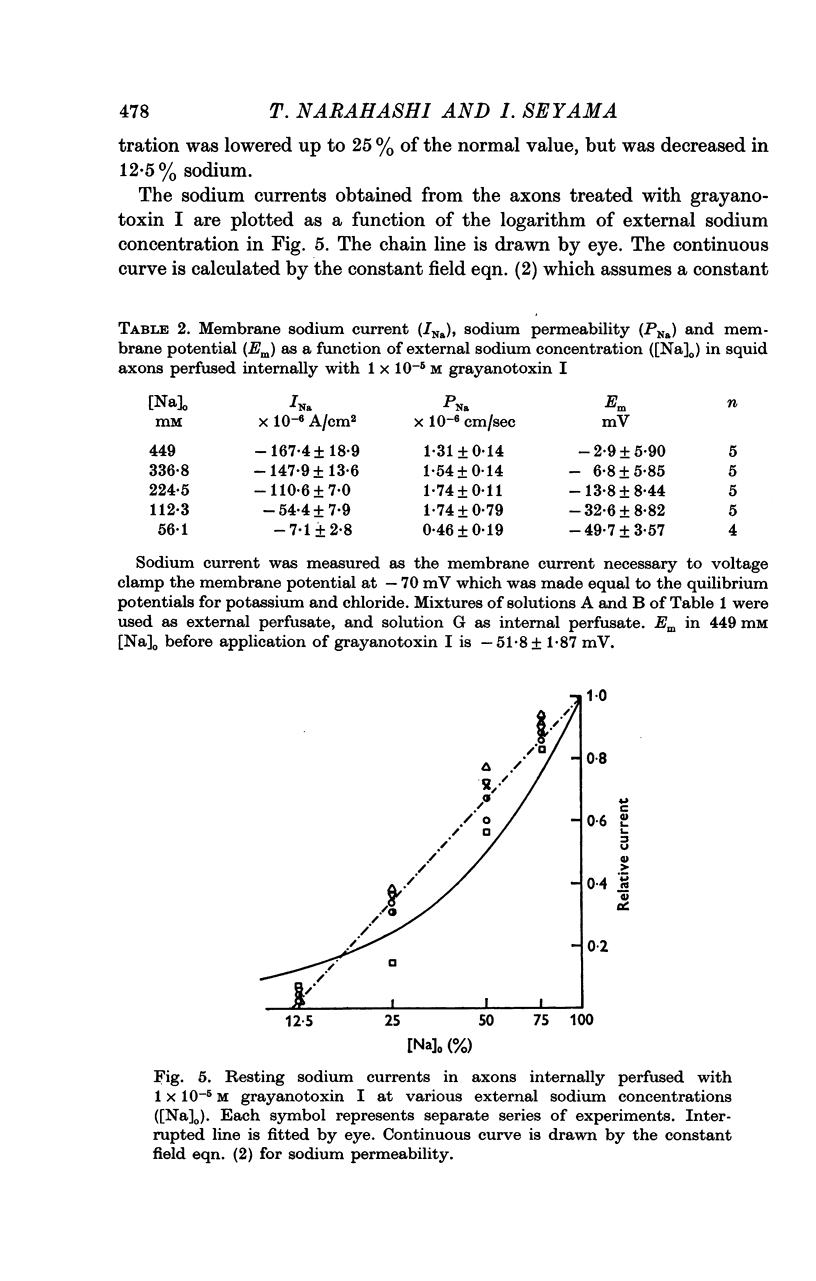
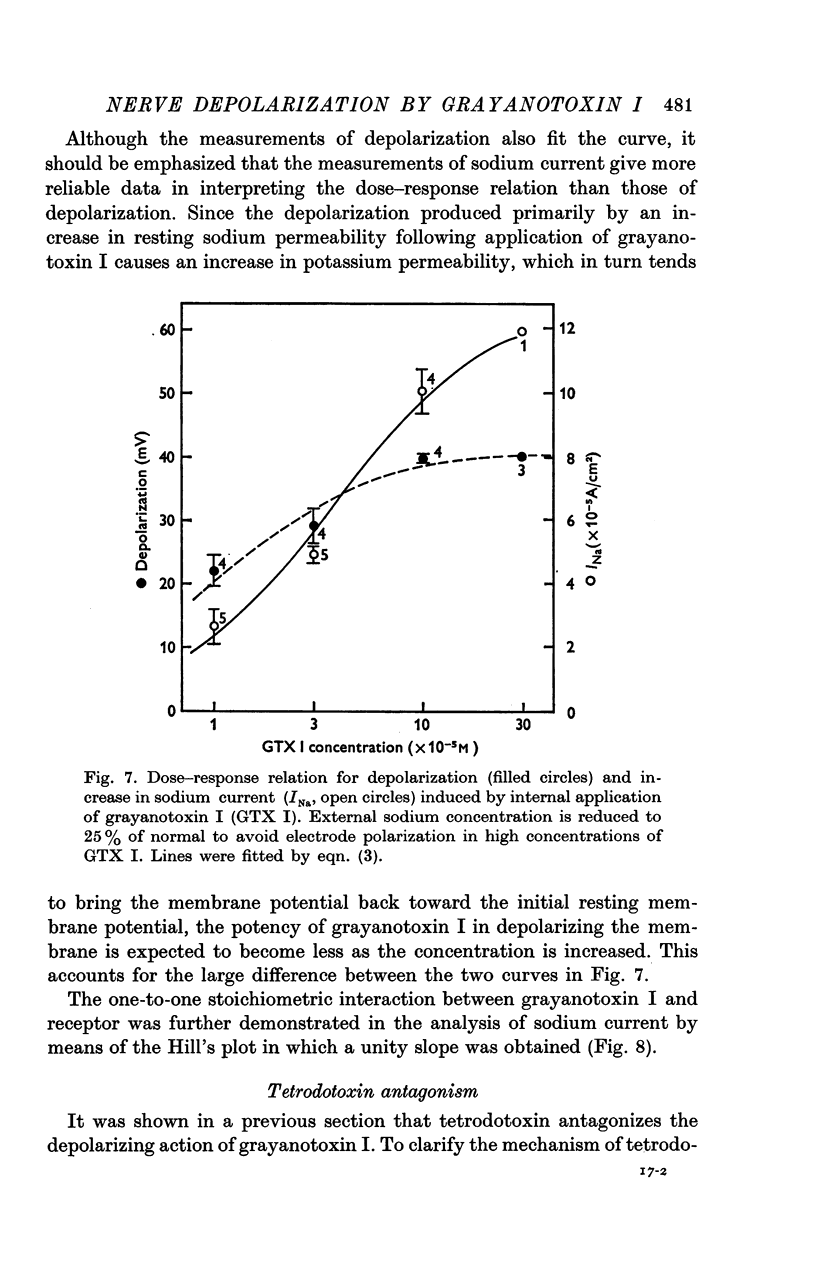
4. The resting sodium permeability as measured by voltage clamp was increased to 1·31 × 10-6 cm/sec by internal application of 1 × 10-5 m grayanotoxin I, an increase by a factor of about 90.
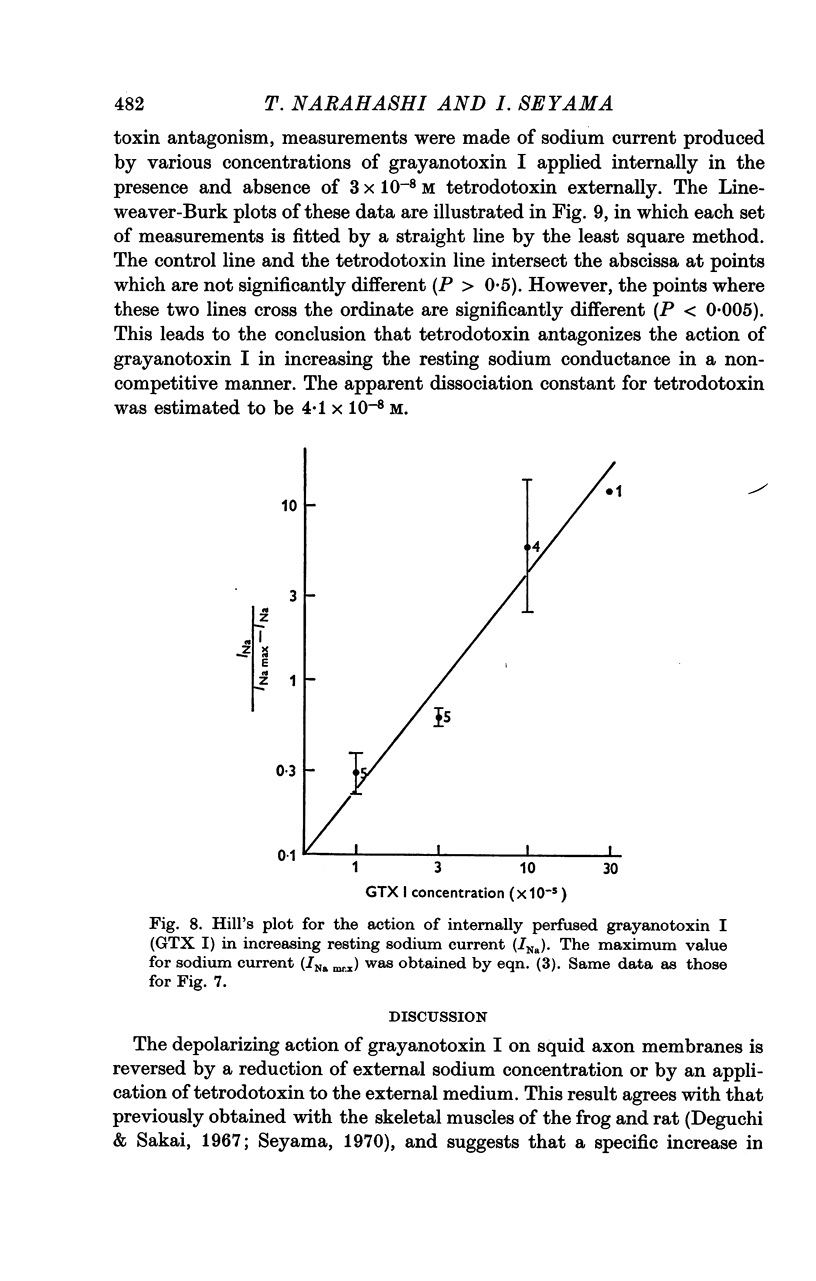
5. The apparent dissociation constant of internally applied grayanotoxin I in increasing the resting sodium permeability was estimated to be 4·12 × 10-5 m, and the toxin interacts with the membrane receptor on a one-to-one stoichiometric basis.
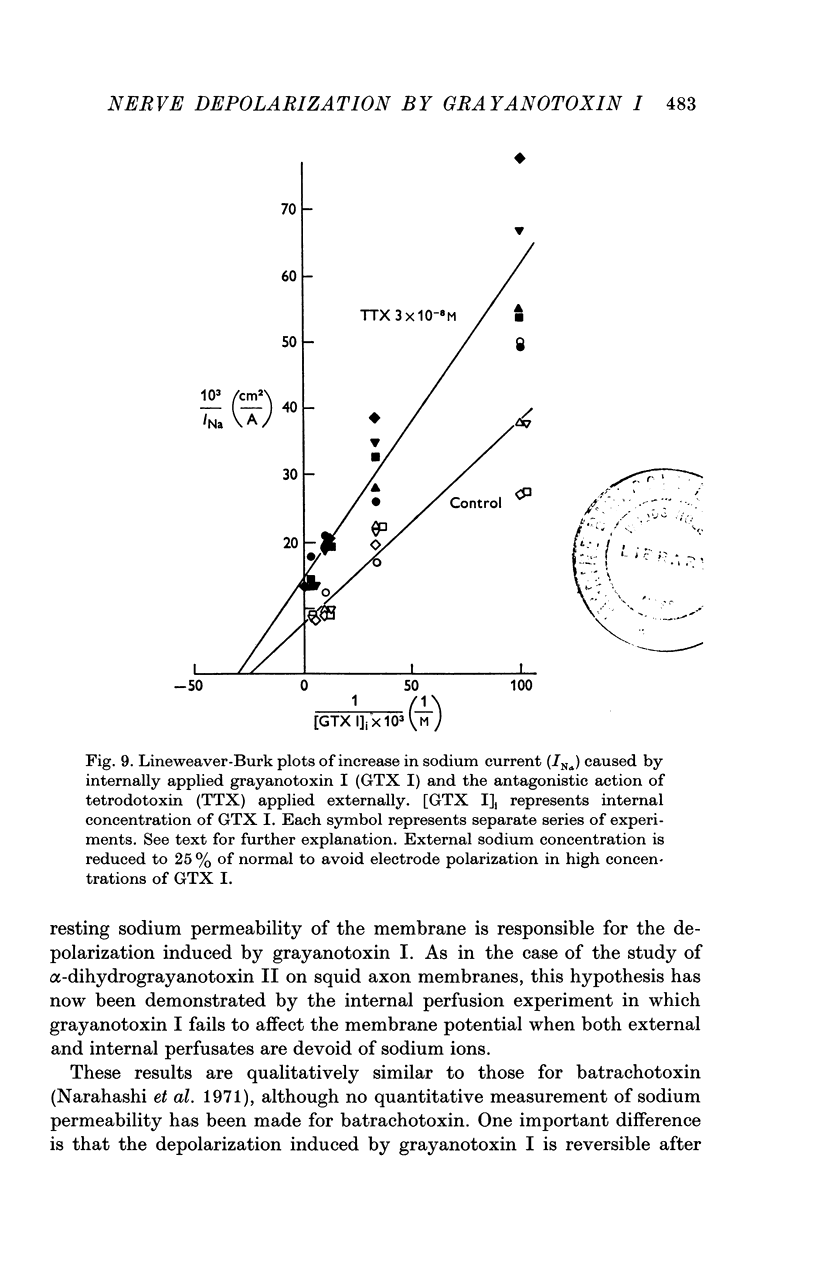
6. Tetrodotoxin antagonized the action of grayanotoxin I in increasing the resting sodium permeability in a non-competitive manner.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADELMAN W. J., Jr, TAYLOR R. E. EFFECTS OF REPLACEMENT OF EXTERNAL SODIUM CHLORIDE WITH SUCROSE ON MEMBRANE CURRENTS OF THE SQUID GIANT AXON. Biophys J. 1964 Nov;4:451–463. doi: 10.1016/s0006-3495(64)86795-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque E. X., Seyama I., Narahashi T. Characterization of batrachotoxin-induced depolarization of the squid giant axons. J Pharmacol Exp Ther. 1973 Feb;184(2):308–314. [PubMed] [Google Scholar]
- BAKER P. F., HODGKIN A. L., SHAW T. I. The effects of changes in internal ionic concentrations on the electrical properties of perfused giant axons. J Physiol. 1962 Nov;164:355–374. doi: 10.1113/jphysiol.1962.sp007026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRINLEY F. J., Jr, MULLINS L. J. ION FLUXES AND TRANSFERENCE NUMBER IN SQUID AXONS. J Neurophysiol. 1965 May;28:526–544. doi: 10.1152/jn.1965.28.3.526. [DOI] [PubMed] [Google Scholar]
- Baker P. F., Blaustein M. P., Keynes R. D., Manil J., Shaw T. I., Steinhardt R. A. The ouabain-sensitive fluxes of sodium and potassium in squid giant axons. J Physiol. 1969 Feb;200(2):459–496. doi: 10.1113/jphysiol.1969.sp008703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Henderson R., Ritchie J. M. The binding of labelled tetrodotoxin to non-myelinated nerve fibres. J Physiol. 1972 Dec;227(1):95–126. doi: 10.1113/jphysiol.1972.sp010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuervo L. A., Adelman W. J., Jr Equilibrium and kinetic properties of the interaction between tetrodotoxin and the excitable membrane of the squid giant axon. J Gen Physiol. 1970 Mar;55(3):309–335. doi: 10.1085/jgp.55.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deguchi T., Sakai Y. Sustained after-depolarization in grayanotoxin-treated muscle cell membrane. Nihon Seirigaku Zasshi. 1967;29(4):172–173. [PubMed] [Google Scholar]
- FRANKENHAEUSER B. Sodium permeability in toad nerve and in squid nerve. J Physiol. 1960 Jun;152:159–166. doi: 10.1113/jphysiol.1960.sp006477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman A. R. Electrophysiological activity of tetrodotoxin on the resting membrane of the squid giant axon. Comp Biochem Physiol A Comp Physiol. 1971 Sep 1;40(1):71–82. doi: 10.1016/0300-9629(71)90148-4. [DOI] [PubMed] [Google Scholar]
- Goldman D. E. POTENTIAL, IMPEDANCE, AND RECTIFICATION IN MEMBRANES. J Gen Physiol. 1943 Sep 20;27(1):37–60. doi: 10.1085/jgp.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):449–472. doi: 10.1113/jphysiol.1952.sp004717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafemann D. R. Binding of radioactive tetrodotoxin to nerve membrane preparations. Biochim Biophys Acta. 1972 May 9;266(2):548–556. doi: 10.1016/0005-2736(72)90110-1. [DOI] [PubMed] [Google Scholar]
- Keynes R. D., Ritchie J. M., Rojas E. The binding of tetrodotoxin to nerve membranes. J Physiol. 1971 Feb;213(1):235–254. doi: 10.1113/jphysiol.1971.sp009379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumazawa Z., Iriye R. Stereochemistry of grayanotoxin-II. Tetrahedron Lett. 1970 Mar;(12):927–930. doi: 10.1016/s0040-4039(01)97868-2. [DOI] [PubMed] [Google Scholar]
- Matsumoto T., Watanabe M. Stereochemistry of grayanotoxin. Tetrahedron Lett. 1968 Dec;(57):6019–6022. doi: 10.1016/s0040-4039(00)70782-9. [DOI] [PubMed] [Google Scholar]
- Moore J. W., Narahashi T., Shaw T. I. An upper limit to the number of sodium channels in nerve membrane? J Physiol. 1967 Jan;188(1):99–105. doi: 10.1113/jphysiol.1967.sp008126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narahashi T., Albuquerque E. X., Deguchi T. Effects of batrachotoxin on membrane potential and conductance of squid giant axons. J Gen Physiol. 1971 Jul;58(1):54–70. doi: 10.1085/jgp.58.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narahashi T., Anderson N. C. Mechanism of excitation block by the insecticide allethrin applied externally and internally to squid giant axons. Toxicol Appl Pharmacol. 1967 May;10(3):529–547. doi: 10.1016/0041-008x(67)90092-0. [DOI] [PubMed] [Google Scholar]
- Narahashi T., Anderson N. C., Moore J. W. Comparison of tetrodotoxin and procaine in internally perfused squid giant axons. J Gen Physiol. 1967 May;50(5):1413–1428. doi: 10.1085/jgp.50.5.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narahashi T., Anderson N. C., Moore J. W. Tetrodotoxin does not block excitation from inside the nerve membrane. Science. 1966 Aug 12;153(3737):765–767. doi: 10.1126/science.153.3737.765. [DOI] [PubMed] [Google Scholar]
- Ota M., Narahashi T., Keeler R. F. Effects of veratrum alkaloids on membrane potential and conductance of squid and crayfish giant axons. J Pharmacol Exp Ther. 1973 Jan;184(1):143–154. [PubMed] [Google Scholar]
- Seyama I. Effect of grayanotoxin 1 on the electrical properties of rat skeletal muscle fibers. Jpn J Physiol. 1970 Aug;20(4):381–393. doi: 10.2170/jjphysiol.20.381. [DOI] [PubMed] [Google Scholar]
- Seyama I., Narahashi T. Increase in sodium permeability of squid axon membranes by -dihydrograyanotoxin II. J Pharmacol Exp Ther. 1973 Feb;184(2):299–307. [PubMed] [Google Scholar]
- Tokuyama T., Daly J., Witkop B. The structure of batrachotoxin, a steroidal alkaloid from the Colombian arrow poison frog, Phyllobates aurotaenia, and partial synthesis of batrachotoxin and its analogs and homologs. J Am Chem Soc. 1969 Jul 2;91(14):3931–3938. doi: 10.1021/ja01042a042. [DOI] [PubMed] [Google Scholar]
- Ulbricht W. The effect of veratridine on excitable membranes of nerve and muscle. Ergeb Physiol. 1969;61:18–71. doi: 10.1007/BFb0111446. [DOI] [PubMed] [Google Scholar]
- Wang C. M., Narahashi T., Scuka M. Mechanism of negative temperature coefficient of nerve blocking action of allethrin. J Pharmacol Exp Ther. 1972 Sep;182(3):442–453. [PubMed] [Google Scholar]
- Wu C. H., Narahashi T. Mechanism of action of propranolol on squid axon membranes. J Pharmacol Exp Ther. 1973 Jan;184(1):155–162. [PubMed] [Google Scholar]


